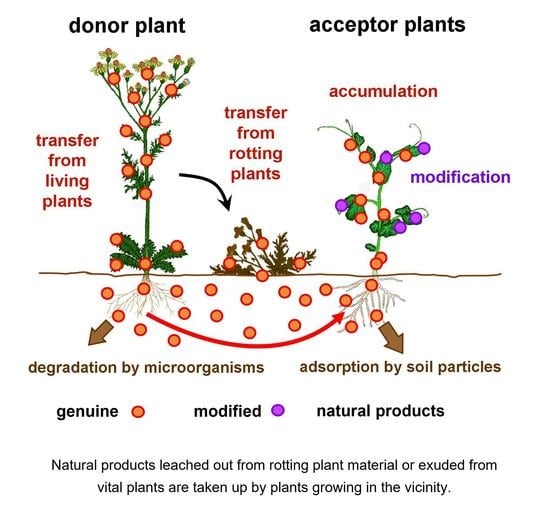
Novel Cognitions in Allelopathy: Implications from the “Horizontal Natural Product Transfer”
Abstract
:1. Background
2. Horizontal Natural Product Transfer—Basic Principles and Cognitions
2.1. Uptake of Chemicals from the Soil
2.2. Horizontal Natural Product Transfer between Vital Plants
2.3. Translocation of Chemicals within the Acceptor Plants
2.4. Modification of the Imported Compounds in the Acceptor Plants
3. Horizontal Natural Product Transfer and Allelopathy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inderjit, K.; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 17, 529–539. [Google Scholar]
- Bertin, C.; Yang, X.; West, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of Allelopathic Substances in Buckwheat (Fagopyrum esculentum Moench). J. Agri. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.J. The Historical Bases of the Concept of Allelopathy. J. Hist. Biol. 1985, 18, 71–102. [Google Scholar] [CrossRef]
- De Meyer, G.; Capieau, K.; Audenaert, K.; Buchala, A.; Métraux, J.P.; Höfte, M. Nanogram Amounts of salicylic acid produced by the Rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean. Mol. Plant Microbe Interact. 1999, 12, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthe, B.; Schulz, M.; Schnabl, H. Effects of salicylic acid on growth and stomatal movements of Vicia faba L.: Evidence for salicylic acid metabolization. J. Chem. Ecol. 1992, 18, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G. Review: Nitrate transporters in plants: Structure, function and regulation. Biochim. Biophys. Acta 2000, 1465, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P.; Takahashi, H.; Hawkesford, M.J. Plant sulphate transporters: Co-ordination of uptake, intracellular and long-distance transport. J. Exp. Bot. 2004, 55, 1765–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapp, S.; Legind, C.N. Uptake of organic contaminants from soil into vegetables and fruits. In Dealing with Contaminated Sites; Swartjes, F.A., Ed.; Springer: Heidelberg, The Netherlands, 2011. [Google Scholar]
- Boxall, A.B.A.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.; Levy, L.S. Uptake of veterinary medicines from soils into plants. J. Agri. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef]
- Inoue, J.; Chamberlain, K.; Bromilow, R.H. Physicochemical factors affecting the uptake by roots and translocation to shoots of amine bases in barley. Pest. Manag. Sci. 1998, 54, 8–21. [Google Scholar] [CrossRef]
- Nwoko, C.O. Trends in phytoremediation of toxic elemental and organic pollutants. Afr. J. Biotechnol. 2010, 9, 6010–6016. [Google Scholar]
- Sibout, R.; Höfte, H. Plant Cell Biology: The ABC of Monolignol Transport. Curr. Biol. 2012, 22, R533–R535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA—European Food Safety Authority. Setting of temporary MRLs for nicotine in tea, herbal infusions, spices, rose hips and fresh herbs. EFSA J. 2011, 9, 2098. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 4908. [Google Scholar] [CrossRef] [Green Version]
- EFSA—European Food Safety Authority. Human acute exposure assessment to tropane alkaloids. EFSA J. 2018, 16, 5160. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Engelhardt, U.H.; Hänsel, S.; Thräne, C.; Nowak, M.; Kleinwächter, M. Nicotine uptake by peppermint plants as a possible source of nicotine in plant-derived products. Agron. Sustain. Dev. 2015, 35, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.; Wittke, C.; Lederer, I.; Klier, B.; Kleinwächter, M.; Selmar, D. Interspecific transfer of pyrrolizidine alkaloids: An unconsidered source of contaminations of phytopharmaceuticals and plant derived commodities. Food Chem. 2016, 213, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Radwan, A.; Abdalla, N.; Taha, H.; Wittke, C.; El-Henawy, A.; Alshaal, T.; Amer, M.; Nowak, M.; El-Ramady, H. Uptake of Nicotine from Discarded Cigarette Butts—A so far unconsidered path of contamination of plant derived commodities. Environ. Pollut. 2018, 238, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.J.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of Pyrrolizidine Alkaloids in food. EFSA Support. Publ. 2015, 12, 859E. [Google Scholar] [CrossRef]
- Selmar, D.; Radwan, A.; Nowak, M. Horizontal Natural Product Transfer: A So Far Unconsidered Source of Contamination of Plant-Derived Commodities. J. Environ. Anal. Toxicol. 2015, 5, 4. [Google Scholar] [CrossRef]
- Nowak, M.; Yahyazadeh, M.; Lewerenz, L.; Selmar, D. Horizontal Natural Product Transfer: A so far unconsidered source of contamination of medicinal. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M.M., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 215–226. [Google Scholar]
- Selmar, D.; Radwan, A.; Hijazin, T.; Abouzeid, S.; Yahyazadeh, M.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Horizontal Natural Product Transfer: Intriguing Insights into a Newly Discovered Phenomenon. J. Agri. Food Chem. 2019, 67, 8740–8745. [Google Scholar] [CrossRef]
- Hijazin, T.; Lewerenz, L.; Yahyazadeh, M.; Selmar, D. Horizontal Natural Product Transfer: A Phenomenon Which Is Responsible for the Widespread Alkaloidal Contaminations of Herbal Products. In Environmental Challenges and Medicinal Plants: Environmental Challenges and Solutions; Aftab, T., Ed.; Springer Nature: Cham, Switzerland, 2022; pp. 183–201. [Google Scholar]
- Hijazin, T.; Radwan, A.; Abouzeid, S.; Dräger, G.; Selmar, D. Uptake and modification of umbelliferone by various seedlings. Phytochemistry 2019, 157, 194–199. [Google Scholar] [CrossRef]
- Abouzeid, S.; Beutling, U.; Selmar, D. Stress-induced modification of indole alkaloids: Phytomodificines as a new category of specialized metabolites. Phytochemistry 2019, 159, 102–107. [Google Scholar] [CrossRef]
- Selmar, D.; Abouzeid, S.; Radwan, A.; Hijazin, T.; Yahyazadeh, M.; Lewerenz, L.; Nowak, M.; Kleinwächter, M. Horizontal natural product transfer—A novel attribution in allelopathy. In Reference Series in Phytochemistry. Co-Evolution of Secondary Metabolite; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 429–439. [Google Scholar]
- Fan, P.; Hostettmann, K.; Lou, H. Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc. (Polygonaceae). Chemoecology 2010, 20, 223–227. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.Z.; Yan, Z.Q.; Guo, H.R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure-activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–279. [Google Scholar] [CrossRef]
- Trapp, S. Modelling uptake into roots and subsequent translocation of neutral and ionizable organic compounds. Pest Manag. Sci. 2000, 56, 767–778. [Google Scholar] [CrossRef]
- Cronin, M.T.D.; Livingstone, J. Calculation of physiochemical properties. In Predicting Chemical Toxicity and Fate; Cronin, T.D., Livingstone, J., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 31–40. [Google Scholar]
- Limmer, M.A.; Burken, J.G. Plant Translocation of Organic Compounds. Molecularand Physicochemical Predictors. Environ. Sci. Technol. Lett. 2014, 1, 156–161. [Google Scholar] [CrossRef]
- Hurtado, C.; Domínguez, C.; Pérez-Babace, L.; Cãnameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grow under controlled conditions”. J. Hazard. Mat. 2016, 305, 139–148. [Google Scholar] [CrossRef]
- Nowak, M.; Selmar, D. Cellular distribution of alkaloids and their translocation via phloem and xylem: The importance of compartment pH. Plant Biol. 2016, 18, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, M.; Nowak, M.; Kima, H.; Selmar, D. Horizontal natural product transfer: A potential source of alkaloidal contaminants in phytopharmaceuticals. Phytomedicine 2017, 34, 21–25. [Google Scholar] [CrossRef]
- Hijazin, T.; Radwan, A.; Lewerenz, L.; Abouzeid, S.; Selmar, D. The uptake of alkaloids by plants from the soil is determined by rhizosphere pH. Rhizosphere 2020, 15, 100234. [Google Scholar] [CrossRef]
- Nakano, H.; Nakajima, E.; Fujii, Y.; Yamada, K.; Shigemori, H.; Hasegawa, K. Leaching of the allelopathic substance, L-tryptophan from the foliage of mesquite (Prosopis juliflora DC.) plants by water spraying. Plant Growth Regul. 2003, 40, 49–52. [Google Scholar] [CrossRef]
- Tukey, H.B. The leaching of substances from plants. Annu. Rev. Plant Biol. 1970, 21, 305–324. [Google Scholar] [CrossRef]
- Pavlović, N.M.; Maksimović, V.; Maksimović, J.D.; Orem, W.H.; Tatu, C.A.; Lerch, H.E.; Bunnell, J.E.; Kostić, E.N.; Szilagyi, D.N.; Paunescu, V. Possible health impacts of naturally occurring uptake of aristolochic acids by maize and cucumber roots: Links to the etiology of endemic (Balkan) nephropathy. Environ. Geochem. Health 2013, 35, 5–226. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, Q.; Chan, W. Uptake and accumulation of nephrotoxic and carcinogenic aristolochic acids in food crops grown in Aristolochia clematitis-contaminated soil and water. J. Agri. Food Chem. 2016, 64, 107–112. [Google Scholar] [CrossRef]
- Letsyo, E.; Adams, Z.S.; Dzikunoo, J.; Asante-Donyinah, D. Uptake and accumulation of pyrrolizidine alkaloids in the tissues of maize (Zea mays L.) plants from the soil of a 4-year-old Chromolaena odorata dominated fallow farmland. Chemosphere 2021, 270, 128669. [Google Scholar] [CrossRef]
- Hazrati, H.; Fomsgaard, I.S.; Kudsk, P. Root-Exuded Benzoxazinoids: Uptake and translocation in neighboring plants. J. Agri. Food Chem. 2020, 68, 10609–10617. [Google Scholar] [CrossRef]
- Jandrić, Z.; Rathor, M.; Ghhem-Kieth, S.; Adu-Gyamfi, J.; Mayr, L.; Resch, C.; Bado, S.; Švarc-Gajić, J.; Cannavan, A. Uptake of 14C-atropine and/or its transformation products from soil by wheat (Triticum aestivum var Kronjet) and their translocation to shoots. J. Environ. Sci. Health B 2013, 48, 1034–1104. [Google Scholar] [CrossRef] [PubMed]
- García-Jorgensen, D.B.; Hansen, H.C.B.; Abrahamsen, P.; Diamantopoulos, E. A novel model concept for modelling the leaching of natural toxins: Results for the case of ptaquiloside. Environ. Sci. Process. Impacts 2020, 22, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Matile, P. Localization of alkaloids and mechanism of their accumulation in vacuoles of Chelidonium majus laticifers. Nova Acta Leopold. 1976, 7, 139–156. [Google Scholar]
- Waldhauser, S.S.M.; Baumann, T.W. Compartmentation of caffeine and related purine alkaloids depends exclusively on the physical chemistry of their vacuolar complex formation with chlorogenic acids. Phytochemistry 1996, 42, 985–996. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Vivanco, J.M. Root specific elicitation and exudation of fluorescent β-carbolines in transformed root cultures of Oxalis tuberosa. Plant Physiol. Biochem. 2003, 41, 345–353. [Google Scholar] [CrossRef]
- Ruiz-May, E.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Differential secretion and accumulation of terpene indole alkaloids in hairy roots of Catharanthus roseus treated with methyl jasmonate. Mol. Biotechnol. 2009, 41, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Agblevor, F.A.; Ritesh, K.C.; Jelesko, J.G. Enhanced production of the alkaloid nicotine in hairy root cultures of Nicotiana tabacum. Plant Cell Tissue Organ Cult. 2013, 113, 121–129. [Google Scholar] [CrossRef]
- Toppel, G.; Witte, L.; Riebesehl, B.; Borstel, K.V.; Hartmann, T. Alkaloid patterns and biosynthetic capacity of root cultures from some pyrrolizidine alkaloid producing Senecio species. Plant Cell Rep. 1987, 6, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N.; Bazin, I.; Dan, K.; Obata, K.; Kigawa, K.; Ueda, K.; Sato, F.; Forestier, C.; Yazaki, K. Involvement of CjMDR1, a plant MDR-type ABC protein, in alkaloid transport in Coptis japonica. Proc. Natl. Acad. Sci. USA 2003, 100, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otani, M.; Shitan, N.; Sakai, K.; Martinoia, E.; Sato, F.; Yazaki, K. Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica. Plant Physiol. 2005, 138, 1939–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.E.; Inzé, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.-M.; Moriyama, Y.; Yazaki, K. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazaki, K.; Sugiyama, A.; Morita, M.; Shitan, N. Secondary transport as an efficient membrane transport mechanism for plant secondary metabolites. Phytochem. Rev. 2008, 7, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Baumann, T.W.; Gabriel, H. Metabolism and excretion of caffeine during germination of Coffea arabica L. Plant Cell Physiol. 1984, 25, 1431–1436. [Google Scholar] [CrossRef]
- Schulz, M.; Knop, M.; Kant, S.; Sicker, D.; Voloshchuk, N.; Gryganski, A. Detoxification of allelochemicals- the case of bezoxazolin-2(3H)-one(BOA). In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, R.M.J., Pedrol, N., González, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 157–170. [Google Scholar]
- Bais, H.P.; Park, S.W.; Stermitz, F.R.; Halligan, K.M.; Vivanco, J.M. Exudation of fluorescent β-carbolines from Oxalis tuberosa L. roots. Phytochemistry 2002, 61, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Hama, J.; Strobel, B.W. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ. Sci. Eur. 2020, 32, 126. [Google Scholar] [CrossRef]
- Hama, J.; Strobel, B.W. Occurrence of pyrrolizidine alkaloids in ragwort plants, soils and surface waters at the field scale in grassland. Sci. Total Environ. 2021, 755, 142822. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.C.; Marxmiller, R.L.; Yang, A.Y.S. Study of root uptake and xylem translocation of cinmethylin and related compounds in detopped soybean roots using a pressure chamber technique. Plant Physiol. 1990, 93, 1573–1578. [Google Scholar] [CrossRef] [Green Version]
- Singh, S. Guttation: Mechanism, Momentum and Modulation. Bot. Rev. 2016, 82, 149–182. [Google Scholar] [CrossRef]
- Ehmke, A.; Borstel, K.V.; Hartmann, T. Alkaloid N-oxides as transport and vacuolar storage compounds of pyrrolizidine alkaloids in Senecio vulgaris L. Planta 1988, 176, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Dierich, B. Chemical diversity and variation of pyrrolizidine alkaloids of the senecionine type: Biological need or coincidence? Planta 1989, 206, 443–451. [Google Scholar] [CrossRef]
- Witte, L.; Ehmke, A.; Hartmann, T. Interspecific flow of pyrrolizidine alkaloids: From plants via aphids to ladybirds. Naturwissenschaften 1990, 77, 540–543. [Google Scholar] [CrossRef]
- Riedell, W.E.; Schumacher, T.E. Transport of water and nutrients in plants. In Agricultural Sciences; Lal, R., Ed.; EOLSS Publications: Abu Dhabi, United Arab Emirates, 2009; Volume 1, pp. 371–387. [Google Scholar]
- Sandermann, H. Higher plant metabolism of xenobiotics: The ´green liver‘ concept. Pharmacogenetics 1994, 44, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Schäffner, A.; Messener, B.; Langebartels, C.; Sandermann, H. Genes and enzymes for in-planta phytoremediation of air, water and soil. Acta Biotechnol. 2002, 22, 141–151. [Google Scholar] [CrossRef]
- Burken, J.G. Uptake and metabolism of organic compounds: Green-liver model. In Phytoremediation: Transformation and Control of Contaminants; McCutcheon, S.C., Schnoor, J.L., Eds.; Wiley: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Ronczka, S.; These, A.; Bodi, D.; Preiß-Weigert, A. International collaborative study for the determination of pyrrolizidine alkaloids in honey and herbal tea by SPE-LC-MS/MS. BfR Wiss. 2015, 1, 1–91. [Google Scholar]
- Cramer, L.; Schiebel, H.-M.; Ernst, L.; Beuerle, T. Pyrrolizidine alkaloids in the food chain. Development, validation, and application of a new HPLC-ESI-MS/MS sum parameter method. J. Agri. Food Chem. 2013, 61, 11382–11391. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.O.; Coulter, S.; Tukey, R.H.; Veronese, M.E.; Birkett, D.J. Cytochromes P450, 1A2, and 2C9 are responsible for the human hepatic O-demethylation of R- and S-naproxen. Biochem. Pharmacol. 1996, 51, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, L.; Hijazin, T.; Abouzeid, S.; Hänsch, R.; Selmar, D. Pilot study on the uptake and modification of harmaline in acceptor plants: An innovative approach to visualize the interspecific transfer of natural products. Phytochemistry 2020, 174, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Blum, U. Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: Nutrient culture studies. J. Chem. Ecol. 2005, 31, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Boydston, R.A.; Morra, M.J.; Borek, V.; Clayton, L.; Vaughn, S.F. Onion and weed response to mustard (Sinapis alba ) seed meal. Weed Sci. 2011, 59, 546–552. [Google Scholar] [CrossRef]
- Tavella, L.B.; Silva, P.S.L.; Monteiro, A.L.; de Oliveira, V.R.; de Oliveira-Fernandes, P.L. Gliricidia sepium intercropping for weed management in immature corn ear production. Cien. Agron. 2017, 48, 50–656. [Google Scholar] [CrossRef]
- Avato, P.; D’Addabbo, T.; Leonetti, P.; Argentieri, M.P. Nematicidal potential of Brassicaceae. Phytochem. Rev. 2013, 12, 791–802. [Google Scholar] [CrossRef]
- Singh, A.; Weisser, W.W.; Hanna, R.; Houmgny, R.; Zytynska, S.E. Reduce pests, enhance production: Benefits of intercropping at high densities for okra farmers in Cameroon. Pest Manag. Sci. 2017, 73, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M. Effect of preceding and intercropping crops on suppression of lentil damping-off and root rot disease in New Valley—Egypt. Crop Prot. 2012, 32, 41–46. [Google Scholar] [CrossRef]
- Kocaçalikan, I.; Terzi, I. Allelopathic effects of walnut leaf extracts and juglone on seed germination and seedling growth. J. Hort. Sci. Biotechnol. 2001, 76, 436–440. [Google Scholar] [CrossRef]
- Hadrami, A.; Kone, D.; Lepoivre, P. Effect of Juglone on Active Oxygen Species and Antioxidant Enzymes in Susceptible and Partially Resistant Banana Cultivars to Black Leaf Streak Disease. Eur. J. Plant Pathol. 2005, 113, 241–254. [Google Scholar] [CrossRef]
- Sytykiewicz, H. Expression patterns of glutathione transferase gene (GstI) in maize seedlings under juglone-induced oxidative stress. Int. J. Mol. Sci. 2011, 12, 7982–7995. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Wieland, I. Variation in metabolism of BOA among species in various field communities—Biochemical evidence for co-evolutionary processes in plant communities? Chemoecology 1999, 9, 133–141. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; Xiao, C.L.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Amari, L.D.G.E.; Cherif, M.; Kouakou, T.H.; Camara, B.; Konné, D. Salicylic acid and acibenzolar-s-methyl induced resistance against toxic effect of juglone, a toxin of mycosphaerella fijiensis causal agent of banana black leaf streak disease. J. Adv. Agric. 2015, 3, 204–217. [Google Scholar] [CrossRef]
- Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]

| Donor Plant | Type of Alkaloid | Acceptor Plant | Authors |
|---|---|---|---|
| Chromolaena odorata | Pyrrolizidine alkaloids | Zea mays | [44] |
| Secale cereale | Benzoxazinoids | Vicia villosa | [45] |
| Senecio jacobaea | Pyrrolizidine alkaloids | Matricaria chamomilla | [20,22] |
| Senecio jacobaea | Pyrrolizidine alkaloids | Melissa officinalis | [20,22] |
| Senecio jacobaea | Pyrrolizidine alkaloids | Mentha × piperita | [20,22] |
| Senecio jacobaea | Pyrrolizidine alkaloids | Petroselinum crispum | [20,22,25] |
| Senecio jacobaea | Pyrrolizidine alkaloids | Tropaeolum majus | [22] |
| Solanaceae | Atropine | Triticum aestivum | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewerenz, L.; Abouzeid, S.; Yahyazadeh, M.; Hijazin, T.; Selmar, D. Novel Cognitions in Allelopathy: Implications from the “Horizontal Natural Product Transfer”. Plants 2022, 11, 3264. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233264
Lewerenz L, Abouzeid S, Yahyazadeh M, Hijazin T, Selmar D. Novel Cognitions in Allelopathy: Implications from the “Horizontal Natural Product Transfer”. Plants. 2022; 11(23):3264. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233264
Chicago/Turabian StyleLewerenz, Laura, Sara Abouzeid, Mahdi Yahyazadeh, Tahani Hijazin, and Dirk Selmar. 2022. "Novel Cognitions in Allelopathy: Implications from the “Horizontal Natural Product Transfer”" Plants 11, no. 23: 3264. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233264