Study on Flavonoids and Bioactivity Features of Pericarp of Citrus reticulata “Chachi” at Different Harvest Periods
Abstract
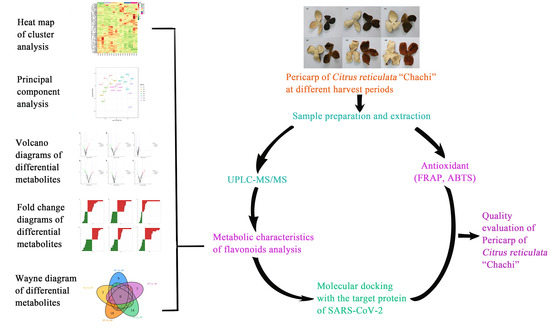
:1. Introduction
2. Results
2.1. Determination of Total Flavone Content
2.2. Metabolic Characteristics of Flavonoids
2.2.1. Qualitative and Quantitative Analysis of Flavonoid Metabolites
2.2.2. Multivariate Analysis of Metabolites
2.3. Identification, Screening, and Analysis of Flavonoid Metabolites
2.4. Analysis of Main Flavonoid Monomers
2.5. Evaluation of Total Antioxidant Capacity
2.6. Molecular Docking Analysis
2.7. KEGG Annotation and Enrichment Analysis of Flavonoid Differential Metabolites
3. Discussion
3.1. Harvesting Periods Affected the Accumulation of Bioactive Components in PCR
3.2. The Difference in Bioactive Components of PCR in Different Harvest Periods Affected Its Antioxidant and Antiviral Effects
3.3. Differences in Flavonoid Metabolites between PCRs Harvested in Different Growth Stages
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Preparation
4.2.1. Sample Preparation and Extraction for Flavonoid Metabolomic Analysis
4.2.2. Quality Control (QC) Samples
4.3. Methods
4.3.1. Determination of Total Flavonoid Content and Total Antioxidant Activity
4.3.2. HPLC Conditions
4.3.3. ESI-Q TRAP-MS/MS
4.4. Data Analysis
4.5. Kyoto Encyclopaedia of Genes and Genomes (KEGG) Annotation and Metabolism Pathway Analysis of Differential Flavonoid Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, One Sections; China Medical Science and Technology Press: Beijing, China, 2020; pp. 199–200. [Google Scholar]
- Luo, Y.; Zeng, W.; Huang, K.E.; Li, D.X.; Chen, W.; Yu, X.Q.; Ke, X.H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ as well as the Citrus reticulata ‘Chachi’ within different storage years using ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J. Pharmaceut. Biomed. 2019, 171, 218–231. [Google Scholar]
- Fu, M.; An, K.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; Xiao, G.; Ti, H. Effects of different temperature and humidity on bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulata (Citrus reticulata ‘Chachi’). LWT-Food Sci. Technol. 2018, 93, 167–173. [Google Scholar] [CrossRef]
- Liang, S.; Wen, Z.; Tang, T.; Liu, Y.; Dang, F.; Xie, T.; Wu, H. Study on flavonoid and bioactivity features of the pericarp of Citri Reticulatae ‘chachi’ during storage. Arab. J. Chem. 2022, 15, 103653–103666. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Zeng, W.; Luo, Y.; Huang, K.; Chen, W.; Yu, X.; Li, D.; Ke, X. Serum Metabolomics of Hyperlipidemia Intervened by Citri Reticulatae Chachiensis Pericarpium. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020, 31, 72–79. [Google Scholar]
- Tian, C.; Xu, H.; Li, J.; Han, Z. Characteristics and intestinal immunomodulating activities of water-soluble pectic polysaccharides from Chenpi with different storage periods. J. Sci. Food Agric. 2018, 98, 3752–3757. [Google Scholar] [CrossRef]
- Guo, J.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Aged citrus peel (chenpi) extract reduces lipogenesis in differentiating 3T3-L1 adipocytes. J. Funct. Foods 2017, 34, 297–303. [Google Scholar] [CrossRef]
- Chen, J.; Chen, A.; Huang, H.; Ye, X.; Rollyson, W.D.; Perry, H.E.; Brown, K.C.; Rojanasakul, Y.; Rankin, G.O.; Dasgupta, P.; et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef] [Green Version]
- Akao, Y.; Ohguchi, K.; Iinuma, M.; Iinuma, M.; Nozawa, Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg. Med. Chem. 2008, 16, 2803–2810. [Google Scholar] [CrossRef]
- Mei, Q.; Lin, H.; Song, Y.; Zhao, Z.; Yang, D. Pharmacological Actions and Clinical Research Progress of Guangchenpi. Eval. Anal. Drug-Use. Hosp. China 2019, 19, 899–902. [Google Scholar]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Jiang, J.; Song, H.; Qin, T.; Li, Z.; Zhang, D.; Huang, S.; Shu, Y.; Xu, J.; Jiang, S.; et al. Predictive analysis and countermeasures in response to COVID-19 epidemic in 2020–2021. Dis. Surveill. 2020, 35, 1068–1072. (In Chinese) [Google Scholar]
- Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine Association. An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19). Chin. J. Viral Dis. 2020, 10, 86–92. [Google Scholar]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Wu, C.; Xu, B.; Li, Z.J.; Song, P.P.; Chao, Z.M. Optimization of the Operations for Storage and Packaging of Citri Reticulatae Pericarpium. Mod. Chin. Med. 2021, 23, 1276–1281. [Google Scholar]
- Bai, G.; Sun, Y.; Wang, Y.; Li, Y.; Ni, Y. A survey of the comparative study of different usage of traditional Chinese medicine and thoughts on its quality control. J. Chin. Med. Mater. 2017, 40, 504–508. [Google Scholar]
- Nair, A.S.; Kurup, R.S.R.; Nair, A.S.; Baby, S. Citrus peels prevent cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef]
- Huang, S.H.; Liang, H.M.; Peng, M.; Jing, Y.T. Determination of Citri reticulatae Pericarpium in Xinhui District. Food Drug 2016, 18, 195–198. (In Chinese) [Google Scholar]
- Pan, J. GC-MS analysis of the changes of volatile oil components in Citri Reticulatae Pericarpium in different harvesting periods. Guide. China Med. 2011, 9, 258–259. [Google Scholar]
- Zeng, S.L.; Li, S.Z.; Lai, C.J.S.; Wei, M.Y.; Chen, B.J.; Li, P.; Zheng, G.D.; Liu, E.H. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae Pericarpium from different harvest time. Phytomedicine 2018, 43, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, G.; Guo, X.; Abbasi, A.M.; Liu, R.H. Influence of the stage of ripeness on the phytochemical profiles, antioxidant and antiproliferative activities in different parts of Citrus reticulata Blanco cv. Chachiensis. LWT-Food Sci. Technol. 2016, 69, 67–75. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Y.; Xu, X.Y.; Chen, W.Q.; Zhao, C.Q.; Liang, J.J.; Ding, Y.Q.; Luo, J.W. Analysis of Deoxynivalenol and Related Derivatives and Metabolites in Cereal and Cereal Products by Ultra Performance Liquid Chromatography-Quadrupole Linear Ion Trap Mass Spectrometry with Isotopic Dilution. Chin. J. Anal Chem. 2016, 44, 1728–1734. [Google Scholar]
- Liang, X.; Gou, Z.; Zhu, X.; Wang, Y.; Li, N.; Fu, X.; Du, S.; Feng, P.; Qin, Y. Characterization of drug-related impurities using UPLC-QTrap-MS/MS in three scan modes: Application to daptomycin. Int. J. Mass Spectrom. 2021, 468, 116657. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Chen, H.; Chen, S.; Liu, Y. Analysis of Flavonoid Metabolites in Citrus Peels (Citrus reticulata ‘Dahongpao’) Using UPLC-ESI-MS/MS. Molecules 2019, 24, 2680. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, X.; Qu, J.; Zhang, L.; Zhang, Y.; Qu, X.; Huang, Z.; Xu, W. Comprehensive Comparison on the Chemical Profile of Guang Chen Pi at Different Ripeness Stages Using Untargeted and Pseudotargeted Metabolomics. J. Agric. Food Chem. 2020, 68, 8483–8495. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, B.; Yi, D.; Chen, H.P.; Yang, F.Q.; Wang, F.; Liu, Y.P. Integration of high throughput sequencing and widely targeted metabolomics reveals the aging mechanism of Pericarpium Citri Reticulatae ‘Chachiensis’. Nat. Prod. Res. Dev. 2022, 34, 553–562. [Google Scholar]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.W.; Jiang, L.; Zheng, G.D. Study on the Content of Flavonoids in Citrus reticulata ‘Chachi’ from Various Habitats and Different Collecting Periods. J. Chin. Med. Mater. 2010, 33, 173–176. [Google Scholar]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional regulation of secondary metabolite biosynthesis in plants. BBA-Gene Regul. Mech. 2013, 1829, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.F.; Almeida, M.P.; Leite, M.F.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, J.M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2019, 273, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Cen, Z.; Fu, Y.; Zhu, X.; He, H.; Kong, D.; Wu, H. The variation in essential oils composition, phenolic acids and flavonoids is correlated with changes in antioxidant activity during Cinnamomum loureirii bark growth. Arab. J. Chem. 2021, 14, 103249. [Google Scholar] [CrossRef]
- Zvaigzne, G.; Kārkliņa, D. Health Promoting Chemical Components of Orange Juice. Proc. Latv. Acad. Sci. Sect. B Nat. Exact. Appl. Sci. 2013, 67, 329–333. [Google Scholar] [CrossRef]
- Cui, L.; Song, Y.; Miao, M. Possible Mechanism of Citri Reticulatae Pericarpium Intervening on COVID-19 Based on Network Pharmacology and Molecular Docking. Pharmacol. Clin. Chin. Mater. Med. 2020, 36, 28–33. [Google Scholar]
- Liu, C.; Ma, Q.; Wang, X.; Lu, Q.; Liu, X.; Yang, Y.; Rong, R. Study on Efficient Separation of Polymethoxylated Flavones from Citri Reticulatae Pericarpium and their Potential Inhibitory Activity Against SARS-CoV-2 3CLpro. Mod. Tradit. Chin. Med. Mater. Med.-World Sci. Technol. 2021, 23, 4622–4631. (In Chinese) [Google Scholar]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; De Vos, C.H.R.; Van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonoids. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xia, R. Expression of chalcone synthase and chalcone isomerase genes and accumulation of corresponding flavonoids during fruit maturation of Guoqing No. 4 satsuma mandarin (Citrus unshiu Marcow). Sci. Hortic. 2010, 125, 110–116. [Google Scholar] [CrossRef]
- Frydman, A.; Liberman, R.; Huhman, D.V.; Carmeli-Weissberg, M.; Sapir-Mir, M.; Ophir, R.; Sumner, L.W.; Eyal, Y. The molecular and enzymatic basis of bitter/non-bitter flavor of citrus fruit: Evolution of branch-forming rhamnosyltransferases under domestication. Plant J. 2013, 73, 166–178. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, Y.C.; Hsu, H.W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Li, B.; Liu, J. Optimization of Extraction of Flavonoids from Shaddock Peel by Response Surface Methodology. Adv. J. Food Sci. Technol. 2015, 8, 229–231. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2015, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]














| Harvest Time | Total Antioxidant Capacity | |
|---|---|---|
| FRAP (mM) | ABTS (mM) | |
| Jul | 1.507 ± 0.031 b | 0.458 ± 0.084 ab |
| Aug | 2.167 ± 0.025 d | 0.639 ± 0.027 c |
| Sept | 1.823 ± 0.153 c | 0.631 ± 0.027 c |
| Oct | 1.381 ± 0.125 b | 0.601 ± 0.009 bc |
| Nov | 1.104 ± 0.171 a | 0.448 ± 0.196 ab |
| Dec | 1.109 ± 0.010 a | 0.359 ± 0.022 a |
| Positive Control Drug | Binding Energy (kcal/mol) | |||
|---|---|---|---|---|
| 3CLpro | RdRp | PLpro | Spike Protein | |
| Lopinavir | −6.20 | −10.03 | −6.10 | −11.40 |
| Ritonavir | −6.0 | −8.57 | −6.23 | −9.37 |
| Ribavirin | −5.87 | −7.67 | −7.27 | −7.43 |
| Chloroquine | −6.07 | −6.97 | −4.97 | −7.13 |
| Arbidol | −5.97 | −8.07 | −4.90 | −6.97 |
| No. | Flavonoid Name | Binding Energy (kcal/mol) | No. | Flavonoid Name | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | Isoschaftoside | −9.42 | 14 | Ononin | −7.63 |
| 2 | Vitexin | −8.97 | 15 | Phlorizin | −7.43 |
| 3 | Narirutin | −8.93 | 16 | Gallocatechin | −7.23 |
| 4 | Kaempferin | −8.87 | 17 | Nicotiflorin | −7.17 |
| 5 | Isorhoifolin | −8.77 | 18 | Hesperetin | −7.10 |
| 6 | Quercitrin | −8.73 | 19 | Luteolin | −6.93 |
| 7 | Linarin | −8.70 | 20 | Tangeretin | −6.83 |
| 8 | Neohesperidin | −8.33 | 21 | Nobiletin | −6.80 |
| 9 | Naringenin 7-O-glucoside | −8.17 | 22 | 5,6,7,8,3′,4′ -Hexamethoxyflavanone | −6.70 |
| 10 | Lonicerin | −7.93 | 23 | Apigenin | −6.67 |
| 11 | Naringin | −7.83 | 24 | Tectochrysin | −6.53 |
| 12 | Tiliroside | −7.77 | 25 | Hesperidin | −6.43 |
| 13 | Cynaroside | −7.70 | 26 | Saponarin | −6.27 |
| No. | Flavonoid Name | Binding Energy (kcal/mol) | No. | Flavonoid Name | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | Linarin | −11.97 | 7 | Naringin | −10.83 |
| 2 | Isorhoifolin | −11.73 | 8 | Saponarin | −10.67 |
| 3 | Narirutin | −11.63 | 9 | Hesperetin | −10.43 |
| 4 | Lonicerin | −11.13 | 10 | Neohesperidin | −10.33 |
| 5 | Isoschaftoside | −11.07 | 11 | Gallocatechin | −10.07 |
| 6 | Nicotiflorin | −10.93 |
| No. | Flavonoid Name | Binding Energy (kcal/mol) | No. | Flavonoid Name | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | Neohesperidin | −8.00 | 7 | Kaempferin | −7.50 |
| 2 | Naringin | −7.83 | 8 | Narirutin | −7.50 |
| 3 | Quercitrin | −7.80 | 9 | Nicotiflorin | −7.37 |
| 4 | Isorhoifolin | −7.63 | 10 | Gallocatechin | −7.37 |
| 5 | Linarin | −7.63 | 11 | Cynaroside | −7.30 |
| 6 | Lonicerin | −7.53 |
| No. | Flavonoid Name | Binding Energy (kcal/mol) | No. | Flavonoid Name | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | Isoschaftoside | −13.33 | 4 | Isorhoifolin | −11.70 |
| 2 | Lonicerin | −11.90 | 5 | Hesperidin | −11.70 |
| 3 | Naringin | −11.83 |
| No. | Harvest Time | Interval Time (d) |
|---|---|---|
| A1 | 15 Jul 2017 | 0 |
| A2 | 15 Aug 2017 | 30 |
| A3 | 16 Sept 2017 | 31 |
| A4 | 18 Oct 2017 | 32 |
| A5 | 23 Nov 2017 | 35 |
| A6 | 15 Dec 2017 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Zhang, J.; Liu, Y.; Wen, Z.; Liu, X.; Dang, F.; Xie, T.; Wang, J.; Wang, Z.; Wu, H. Study on Flavonoids and Bioactivity Features of Pericarp of Citrus reticulata “Chachi” at Different Harvest Periods. Plants 2022, 11, 3390. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233390
Liang S, Zhang J, Liu Y, Wen Z, Liu X, Dang F, Xie T, Wang J, Wang Z, Wu H. Study on Flavonoids and Bioactivity Features of Pericarp of Citrus reticulata “Chachi” at Different Harvest Periods. Plants. 2022; 11(23):3390. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233390
Chicago/Turabian StyleLiang, Shejian, Jiongbin Zhang, Yufang Liu, Zhijia Wen, Xinxin Liu, Fengliang Dang, Tianxiao Xie, Jingxin Wang, Zhanqian Wang, and Hong Wu. 2022. "Study on Flavonoids and Bioactivity Features of Pericarp of Citrus reticulata “Chachi” at Different Harvest Periods" Plants 11, no. 23: 3390. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11233390