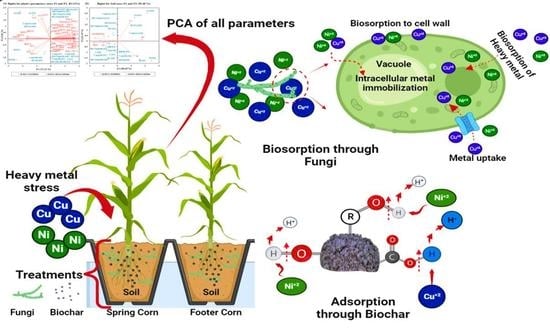
Trichoderma asperellum L. Coupled the Effects of Biochar to Enhance the Growth and Physiology of Contrasting Maize Cultivars under Copper and Nickel Stresses
Abstract
:1. Introduction
2. Results
2.1. Cu, Ni and Pb Removal through Fungal Strains in Aqueous Media
2.2. Effect of T. asperellum and Biochar on Growth and Physiology of Maize Cultivars
2.3. Principal Component Analysis (PCA)
3. Discussion
3.1. Impact of Fungal Strains on Metal Removal
3.2. Maize Growth and Physiology under Biochar, T. asperellum and HM Stresses
3.3. Maize Ionic Homeostasis under Biochar, T. asperellum and HM Stresses
3.4. Impact of Biochar and T. asperellum on Maize ROS and Antioxidant Enzymes under HM Stresses
4. Materials and Methods
4.1. Preparation and Handling of Microbial Isolates
4.2. Lab Trial (Study 1)
4.2.1. Biosorption Trial of Fungal Isolates
4.2.2. Removal Efficiency %
4.2.3. HM Removal Efficiency (q) Per Unit Mass of Fungi
4.3. Preparation of Rice Straw Biochar
4.4. Greenhouse Trial (Study 2)
4.5. Measurements and Analysis
4.5.1. Maize Physiological Characteristics
4.5.2. Maize Biochemical Characteristics
4.5.3. Estimation of Cu and Ni in Plants and Soil
4.5.4. Estimation of Maize Malondialdehyde and Hydrogen Peroxide Concentration
4.5.5. Estimation of Antioxidant Enzyme Activity
4.5.6. Physiochemical Analysis of Soil
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Calviño, D.; Bååth, E. Interaction between pH and Cu toxicity on fungal and bacterial performance in soil. Soil Biol. Biochem. 2016, 96, 20–29. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-Hendawy, S.E. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef] [PubMed]
- Dad, F.; Khan, W.-U.; Tanveer, M.; Ramzani, P.; Shaukat, R.; Muktadir, A. Influence of iron-enriched biochar on Cd sorption, its ionic concentration and redox regulation of radish under cadmium toxicity. Agriculture 2020, 11, 1. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Parvin, K.; Hawrylak-Nowak, B.; Fujita, M. Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.D.; Wey, M.Y.; Chang, S.H. Study on Pb and PAHs emission levels of heavy metals-and PAHs-contaminated soil during thermal treatment process. Int. J. Environ. Eng. 2010, 136, 112–118. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, L.; Yuan, Y.; Liu, Z.; Huang, H.; Tan, F.; Qiu, R. Influence of soil washing with a chelator on subsequent chemical immobilization of heavy metals in a contaminated soil. J. Hazard. Mater. 2010, 178, 578–587. [Google Scholar] [CrossRef]
- Siddiquee, S.; Rovina, K.; Al Azad, S. Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: A review. J. Microb. Biochem. Technol. 2015, 7, 384–395. [Google Scholar] [CrossRef]
- da Rocha Ferreira, G.L.; Vendruscolo, F.; Filho, N.R.A. Biosorption of hexavalent chromium by Pleurotus ostreatus. Heliyon 2019, 5, e01450. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.C. Biosorption: Agreen approach for heavy metals removal from water and waste waters. RJLBPCS 2018, 4, 59. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, S.; Thakkar, A.; Baldi, A.; Murthy, R.S.R.; Sharma, R.K. Physical, chemical and phytoremediation technique for removal of heavy metals. J. Heavy Met. Toxic. Dis. 2016, 1, 2. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Madadi, R.; Bester, K. Fungi and biochar applications in bioremediation of organic micropollutants from aquatic media. Mar. Pollut. Bull. 2021, 166, 112247. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, G.; Dwivedi, S.K. Arsenic and Nickel Stress Response, Their Bioremediation Potential and Mechanism of Trichoderma lixii Isolated from Electroplating Wastewater. Geomicrobiol. J. 2022, 39, 186–198. [Google Scholar] [CrossRef]
- Yaghoubian, Y.; Siadat, S.A.; Telavat, M.R.M.; Pirdashti, H.; Yaghoubian, I. Bio-removal of cadmium from aqueous solutions by filamentous fungi: Trichoderma spp. and Piriformospora indica. Environ. Sci. Pollut. Res. 2019, 26, 7863–7872. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Malińska, K.; Golańska, M.; Caceres, R.; Rorat, A.; Weisser, P.; Ślęzak, E. Biochar amendment for integrated composting and vermicomposting of sewage sludge—The effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour. Technol. 2017, 225, 206–214. [Google Scholar] [CrossRef]
- Batool, M.; Rahman, S.U.; Ali, M.; Nadeem, F.; Ashraf, M.N.; Harris, M.; Du, Z.; Khan, W.-U. Microbial-assisted soil chromium immobilization through zinc and iron-enriched rice husk biochar. Front. Microbiol. 2022, 13, 3401. [Google Scholar] [CrossRef]
- Rabiya, U.E.; Ali, M.; Farooq, M.A.; Siddiq, Z.; Alamri, S.A.; Siddiqui, M.H.; Khan, W.-U.-D. Comparative efficiency of silica gel, biochar, and plant growth promoting bacteria on Cr and Pb availability to Solanum melongena L. in contaminated soil irrigated with wastewater. Front. Plant Sci. 2022, 13, 950362. [Google Scholar] [CrossRef]
- de Araujo, A.S.; Blum, L.E.B.; de Figueiredo, C.C. The synergistic effect of sewage sludge biochar and ’Trichoderma harzianum’ on soybean yield. Aust. J. Crop. Sci. 2019, 13, 1183–1187. [Google Scholar] [CrossRef]
- Herliana, O.; Ahadiyat, Y.R.; Cahyani, W. Utilization of biochar and Trichoderma harzianum to promote growth of shallot and remediate lead-contaminated soil. JDMLM 2022, 8, 2743. [Google Scholar] [CrossRef]
- Shafique, F.; Ali, Q.; Malik, A. Effects of heavy metal toxicity on maze seedlings growth traits. J. Humanit. Soc. Manag. Sci. (JHSMS) 2020, 2020, 1. [Google Scholar] [CrossRef]
- Hassan, M.J.; Raza, M.A.; Rehman, S.U.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Shah, G.A.; Peng, Y.; et al. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Wang, X.; Naveed, M.; Mustafa, A.; Ashraf, S.; Samreen, T.; Nadeem, S.; Jamil, M. Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. Int. J. Environ. Res. Public Health 2021, 18, 4461. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, S.; Chen, Y.; Wang, F.; Yang, D.; Ren, J.; Lu, H.; Ping, L.; Chai, Y. Biochar reduces cadmium accumulation in rice grains in a tungsten mining area-field experiment: Effects of biochar type and dosage, rice variety, and pollution level. Environ. Geochem. Health 2019, 41, 43–52. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Rizwan, M.; Ibrahim, M.; Hussain, A.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ. Sci. Pollut. Res. 2019, 26, 5111–5121. [Google Scholar] [CrossRef]
- Qi, L.; Zhao, W. Strontium uptake and antioxidant capacity comparisons of low accumulator and high accumulator oat (Avena sativa L.) genotypes. Int. J. Phytoremediat. 2019, 22, 227–235. [Google Scholar] [CrossRef]
- Maldaner, J.; Steffen, G.P.K.; Missio, E.L.; Saldanha, C.W.; de Morais, R.M.; Nicoloso, F.T. Tolerance of Trichoderma isolates to increasing concentrations of heavy metals. Int. J. Environ. Stud. 2020, 78, 185–197. [Google Scholar] [CrossRef]
- Zotti, M.; Di Piazza, S.; Roccotiello, E.; Lucchetti, G.; Mariotti, M.; Marescotti, P. Microfungi in highly copper-contaminated soils from an abandoned Fe-Cu sulphide mine: Growth responses, tolerance and bioaccumulation. Chemosphere 2014, 117, 471–476. [Google Scholar] [CrossRef]
- Juniors, P.T.E.; Valeria, C.L.; Santiago, P.O.; Mario, R.M.; Gabriela, S.J. Tolerance to oxidative stress caused by copper (Cu) in Trichoderma asperellum To. ISBAB 2020, 29, 101783. [Google Scholar] [CrossRef]
- Mohsenzadeh, F.; Shahrokhi, F. Biological removing of Cadmium from contaminated media by fungal biomass of Trichoderma species. J. Environ. Health Sci. Eng. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, M.; Soliman, A.S.; Moustafa, Z.R.; El-Reheem, K.M.A. Effect of some soil amendments on yield and quality traits of sugar beet (Beta vulgaris L.) under water stress in sandy soil. Egypt. J. Agron. 2017, 40, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S. Potential of Trichoderma sp. in bioremediation: A review. J. Basic Appl. Eng. Res. 2016, 3, 776–779. [Google Scholar]
- Kanwal, S.; Ilyas, N.; Shabir, S.; Saeed, M.; Gul, R.; Zahoor, M.; Batool, N.; Mazhar, R. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 2018, 41, 526–538. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Ahamd, W.; Numan, M.; Shah, M.; Ahmad, I. Phytostabalization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J. Clean. Prod. 2020, 255, 120318. [Google Scholar] [CrossRef]
- Morales, F.; Pavlovič, A.; Abadía, A.; Abadía, J. Photosynthesis in poor nutrient soils, in compacted soils, and under drought. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Switzerland, 2018; pp. 371–399. [Google Scholar] [CrossRef]
- Aubertin, M.L.; Girardin, C.; Houot, S.; Nobile, C.; Houben, D.; Bena, S.; Brech, Y.; Rumpel, C. Biochar-compost interactions as affected by weathering: Effects on biological stability and plant growth. Agronomy 2021, 11, 336. [Google Scholar] [CrossRef]
- Kumar, G.H.; Kumari, J.P. Heavy metal lead influative toxicity and its assessment in phytoremediating plants—A review. Water Air Soil Pollut. 2015, 226, 324. [Google Scholar] [CrossRef]
- Soliman, M.H.; Alnusaire, T.S.; Abdelbaky, N.F.; Alayafi, A.A.M.; Hasanuzzaman, M.; Rowezak, M.M.; El-Esawi, M.; Elkelish, A. Trichoderma-induced improvement in growth, photosynthetic pigments, Proline, and Glutathione levels in Cucurbita pepo seedlings under salt stress. Phyton 2020, 89, 473–486. [Google Scholar] [CrossRef]
- Hafeez, Y.; Iqbal, S.; Jabeen, K.; Shahzad, S.; Jahan, S.; Rasul, F. Effect of biochar application on seed germination and seedling growth of Glycine max (L.) Merr. Under drought stress. Pak. J. Bot. 2017, 49, 7–13. [Google Scholar]
- Hussain, A.; Maitra, J.; Khan, K.A. Development of biochar and chitosan blend for heavy metals uptake from synthetic and industrial wastewater. Appl. Water Sci. 2017, 7, 4525–4537. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Shan, L.; Anjum, S.; Khan, W.-U.; Ronggui, H.; Iqbal, M.; Virk, Z.A.; Kausar, S. Improved quinoa growth, physiological response, and seed nutritional quality in three soils having different stresses by the application of acidified biochar and compost. Plant Physiol. Biochem. 2017, 116, 127–138. [Google Scholar] [CrossRef]
- Tripathi, N.; Choppala, G.; Singh, R.S. Evaluation of modified chitosan for remediation of zinc contaminated soils. J. Geochem. Explor. 2017, 182, 180–184. [Google Scholar] [CrossRef]
- Turnau, K.; Kottke, I. Fungal Activity as Determined by Microscale Methods with Special Emphasis on Interactions with Heavy Metals. Fungal Community Its Organ. Role Ecosyst. 2005, 287, 20. [Google Scholar]
- Zhang, L.; Shang, Z.; Guo, K.; Chang, Z.; Liu, H.; Li, D. Speciation analysis and speciation transformation of heavy metal ions in passivation process with thiol-functionalized nano-silica. Chem. Eng. J. 2019, 369, 979–987. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [Green Version]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma-plant-pathogen interactions for better development of biocontrol applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Naeem, A.; Saifullah; Zia-Ur-Rehman, M.; Akhtar, T.; Zia, M.H.; Aslam, M. Silicon nutrition lowers cadmium content of wheat cultivars by regulating transpiration rate and activity of antioxidant enzymes. Environ. Pollut. 2018, 242, 126–135. [Google Scholar] [CrossRef]
- Loutfi, H.; Pellen, F.; Le Jeune, B.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Interpretation of the bacterial growth process based on the analysis of the speckle field generated by calibrated scattering media. Opt. Express 2020, 28, 28648. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 7852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, W.-U.; Shaukat, R.; Farooq, M.A.; Ashraf, M.N.; Nadeem, F.; Tanveer, M.; Hamid, Y.; Sun, N. Iron-doped biochar regulated soil nickel adsorption, wheat growth, its physiology and elemental concentration under contrasting abiotic stresses. Sustainability 2022, 14, 7852. [Google Scholar] [CrossRef]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- FAO. The Euphrates Pilot Irrigation Project. Methods of Soil Analysis, Gadeb Soil Laboratory (A Laboratory Manual); Food and Agriculture Organization: Rome, Italy, 1947. [Google Scholar]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. A Manual for the West Asia and North Africa Region; ICARDA: Beirut, Lebanon, 2013; p. 143. [Google Scholar]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Robertson, G.P.; Coleman, D.C.; Sollins, P.; Bledsoe, C.S. Standard Soil Methods for Long-Term Ecological Research; Oxford University Press on Demand: New York, NY, USA, 1999; Volume 2. [Google Scholar]
- Du, E.; Dong, D.; Zeng, X.; Sun, Z.; Jiang, X.; de Vries, W. Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci. Total Environ. 2017, 605-606, 764–769. [Google Scholar] [CrossRef]
- Metwally, R.A.; Soliman, S.A.; Latef, A.A.H.A.; Abdelhameed, R.E. The individual and interactive role of arbuscular mycorrhizal fungi and Trichoderma viride on growth, protein content, amino acids fractionation, and phosphatases enzyme activities of onion plants amended with fish waste. Ecotoxicol. Environ. Saf. 2021, 214, 112072. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.k.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Golui, D.; Datta, S.; Dwivedi, B.; Meena, M.; Trivedi, V. Prediction of free metal ion activity in contaminated soils using WHAM VII, baker soil test and solubility model. Chemosphere 2019, 243, 125408. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S. Short-Term Response of Plants Grown under Heavy Metal Toxicity. In Heavy Metals; IntechOpen: London, UK, 2018; Volume 69. [Google Scholar]
- Latef, A.A.H.A.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O.M. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef] [Green Version]
- Jardim-Messeder, D.; Caverzan, A.; Rauber, R.; Cunha, J.R.; Carvalho, F.E.; Gaeta, M.L.; da Fonseca, G.C.; Costa, J.M.; Frei, M.; Silveira, J.A.; et al. Thylakoidal APX modulates hydrogen peroxide content and stomatal closure in rice (Oryza sativa L.). Environ. Exp. Bot. 2018, 150, 46–56. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R. 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol. 2017, 17, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Changes in soil properties and salt tolerance of safflower in response to biochar-based metal oxide nanocomposites of magnesium and manganese. Ecotoxicol. Environ. Saf. 2021, 211, 111904. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A. Abuse of Magic Mushroom, Peyote Cactus, LSD, Khat, and Volatiles. In Critical Issues in Alcohol and Drugs of Abuse Testing; Academic Press: Cambridge, MA, USA, 2019; pp. 477–494. [Google Scholar] [CrossRef]









| Cultivars | Spring Corn | Footer Corn | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | |
| Length (cm) | Shoot | 30 ± 1 gh | 22 ± 0.5 k | 20 ± 0.3 lm | 34 ± 0.5 bc | 35 ± 0.3 a | 33 ± 0.3 cd | 35 ± 0.3 cd | 31 ± 0.3 fg | 28 ± 0.2 i | 33 ± 0.3 de | 34 ± 0.3 bc | 28 ± 0.5 i | 21 ± 0.5 kl | 19 ± 0.3 m | 34 ± 0.5 bc | 33 ± 0.2 bcd | 31 ± 0.3 ef | 33 ± 0.3 cd | 29 ± 0.5 h | 26 ± 0.3 j | 32 ± 0.3 de | 33 ± 0.2 bc |
| Root | 11 ± 0.3 g | 6 ± 0.3 i | 4 ± 0.2 j | 15 ± 0.4 d | 17 ± 0.2 c | 12 ± 0.4 f | 11 ± 0.4 g | 10 ± 0.3 h | 9 ± 0.5 h | 20 ± 0.6 a | 18 ± 0.3 b | 9 ± 0.1 h | 5 ± 0.4 j | 3 ± 0.3 j | 13 ± 0.4 f | 14 ± 0.3 e | 10 ± 0.2 g | 9 ± 0.3 h | 8 ± 0.3 h | 6 ± 0.3 i | 16 ± 0.7 c | 14 ± 0.6 de | |
| F.W (g) | Shoot | 11 ± 0.2 i | 9 ± 0.2 j | 7 ± 0.5 k | 16.6 ± 0.4 cde | 17 ± 0.2 bcd | 15.5 ± 0.6 fg | 14.5 ± 0.4 g | 15.5 ± 0.3 de | 14.3± 0.4 fg | 19 ± 0.3 a | 17 ± 0.5 b | 9 ± 4 i | 7 ± 2.1 k | 5 ± 1.1 l | 14 ± 2.1 fg | 15.5 ± 3.6 ef | 13.4 ± 4.9 g | 12 ± 4.6 h | 14 ± 5.0 fg | 14 ± 4.4 g | 17 ± 5.4 bc | 15 ± 5.2 ef |
| Root | 4 ± 0.2 d | 0.6 ± 0.1 g | 0.5 ± 0.1 gh | 4.5 ± 0.1 b | 4 ± 0.2 b | 4 ± 0.1 bc | 3.6 ± 0.1 d | 3.7 ± 0.1 d | 3 ± 0.1 ef | 5 ± 0.1 a | 4 ± 0.1 b | 3 ± 0.1 ef | 0.4 ± 0.1 gh | 0.3 ± 0.2 h | 4 ± 0.1 b | 3.7 ± 0.3 d | 3 ± 0.2 e | 3 ± 0.1 ef | 3 ± 0.2 ef | 2 ± 0.2 f | 4 ± 0.3 b | 3.5 ± 0.2 cd | |
| D.W (g) | Shoot | 1.8 ± 0.02 fg | 0.63 ± 0.02 h | 0.4 ± 0.02 h | 2.8 ± 0.1 cd | 3.2 ± 0.2 ab | 2.5 ± 0.1 e | 2.2 ± 0.3 e | 2.6 ± 0.3 bc | 2.3 ± 0.2 cd | 3.6 ± 0.1 a | 2.9 ± 0.5 ab | 1.5 ± 0.01 h | 0.41 ± 0.01 h | 0.3 ± 0.01 h | 2.1 ± 0.5 e | 2.3 ± 0.2 e | 1.8 ± 0.4 f | 1.5 ± 0.4 g | 2 ± 0.1 e | 1.8 ± 0.5 e | 2.5 ± 0.3 d | 2 ± 0.2 e |
| Root | 0.8 ± 0.1 gh | 0.7 ± 0.1 ijk | 0.6 ± 0.1 jk | 1.2 ± 0.3 cd | 1.3 ± 0.5 bc | 1.2 ± 0.5 de | 1 ± 0.2 ef | 1.1 ± 0.4 de | 1 ± 0.1 de | 1.5 ± 0.3 ab | 1.4 ± 0.2 a | 0.7 ± 0.1 gh | 0.5 ± 0.1 k | 0.4 ± 0.1 k | 0.9 ± 0.1 fg | 1 ± 0.1 ef | 0.8 ± 0.1 ghi | 0.7 ± 0.1 hij | 1 ± 0.5 ef | 0.9 ± 0.1 fg | 1.3 ± 0.4 ab | 1 ± 0.5 cd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanullah, F.; Khan, W.-u.-D. Trichoderma asperellum L. Coupled the Effects of Biochar to Enhance the Growth and Physiology of Contrasting Maize Cultivars under Copper and Nickel Stresses. Plants 2023, 12, 958. https://0-doi-org.brum.beds.ac.uk/10.3390/plants12040958
Amanullah F, Khan W-u-D. Trichoderma asperellum L. Coupled the Effects of Biochar to Enhance the Growth and Physiology of Contrasting Maize Cultivars under Copper and Nickel Stresses. Plants. 2023; 12(4):958. https://0-doi-org.brum.beds.ac.uk/10.3390/plants12040958
Chicago/Turabian StyleAmanullah, Fatima, and Waqas-ud-Din Khan. 2023. "Trichoderma asperellum L. Coupled the Effects of Biochar to Enhance the Growth and Physiology of Contrasting Maize Cultivars under Copper and Nickel Stresses" Plants 12, no. 4: 958. https://0-doi-org.brum.beds.ac.uk/10.3390/plants12040958