Effect of UV-A Irradiation on Bioactive Compounds Accumulation and Hypoglycemia-Related Enzymes Activities of Broccoli and Radish Sprouts
Abstract
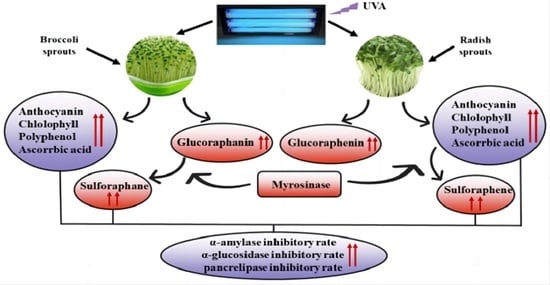
:1. Introduction
2. Results
2.1. Effect of UV-A Irradiation on Growth and Anthocyanin, Chlorophyll Content in Broccoli and Radish Sprouts
2.2. Effect of UV-A Treatment on Soluble Sugar and Soluble Protein Content in Broccoli and Radish Sprouts
2.3. Effect of UV-A Irradiation on Polyphenol and Ascorbic Acid Content in Broccoli and Radish Sprouts
2.4. Effect of UV-A Irradiation on Glucosinolate Content in Broccoli and Radish Sprouts
2.5. Effect of UV-A Irradiation on Myrosinase Activity of Broccoli and Radish Sprouts
2.6. Effect of UV-A Irradiation on Sulforaphane and Sulforaphene Formation in Broccoli and Radish Sprouts
2.7. Effect of UV-A Irradiation on α-Amylase Inhibition Rate in Broccoli and Radish Sprouts
2.8. Effect of UV-A Irradiation on α-Glucosidase Inhibition Rate in Broccoli and Radish Sprouts
2.9. Effect of UV-A Irradiation on Pancrelipase Inhibition Rate in Broccoli and Radish Sprouts
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Cultivation and Treatment
4.2. Determination of Sprout Length, Weight, Anthocyanin and Chlorophyll Content
4.3. Soluble Sugar and Soluble Protein Content Determination
4.4. Ascorbic Acid and Total Phenols Content Analysis
4.5. Glucosinolate Content Determination
4.6. Myrosinase Activity Assay
4.7. Sulforaphane and Sulforaphene Assay
4.8. Hypoglycemia-Related Enzyme Activity Assay
4.8.1. Sample Treatments
4.8.2. α-Amylase Inhibitory Rate
4.8.3. α-Glucosidase Inhibitory Rate
4.8.4. Pancreatic Lipase Inhibitory Rate
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bousquet, J.; Le Moing, V.; Blain, H.; Czarlewski, W.; Zuberbier, T.; de la Torre, R.; Pizarro Lozano, N.; Reynes, J.; Bedbrook, A.; Cristol, J.P.; et al. Efficacy of broccoli and glucoraphanin in COVID-19: From hypothesis to proof-of-concept with three experimental clinical cases. World Allergy Organ. J. 2021, 14, 100498. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Mann, G.E.; Chapple, S.J. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic. Biol. Med. 2018, 122, 150–160. [Google Scholar] [CrossRef]
- Xing, J.J.; Cheng, Y.L.; Chen, P.; Shan, L.; Ruan, R.; Li, D.; Wang, L.J. Effect of high-pressure homogenization on the extraction of sulforaphane from broccoli (Brassica oleracea) seeds. Powder Technol. 2018, 358, 103–109. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. optimization of germination of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and radish (Raphanus sativus L.) seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.J.; Williams, D.J.; Zhang, B.; Wong, L.S.; Jarrett, S.; Pun, S.; Jorgensen, W.; Imsic, M. Radish sprouts versus broccoli sprouts: A comparison of anti-cancer potential based on glucosinolate breakdown products. Acta Hortic. 2009, 841, 187–192. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Chen, Y.; Liu, C.C.; Yang, Y.X. Light regulation of potassium in plants. Plant Physiol. Biochem. 2022, 170, 316–324. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.E.; Zhen, S.; Kim, J. Mild-Intensity UV-A radiation applied over a long duration can improve the growth and phenolic contents of sweet basil. Front. Plant Sci. 2022, 13, 858433. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. UVA, UVB Light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; He, R.; Tan, J.; Liu, K.; Chen, Y.; Liu, H. Effect of supplemental UV-A intensity on growth and quality of kale under red and blue light. Int. J. Mol. Sci. 2022, 23, 6819. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Aboul Naser, A.F.; Zayed, A.; Sharaf El-Dine, M.G. Comparative insights into four major legume sprouts efficacies for diabetes management and its complications: Untargeted versus targeted NMR biochemometrics approach. Metabolites 2023, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Laila, O.; Murtaza, I.; Muzamil, S.; Imtiyaz Ali, S.; Abid Ali, S.; Ahamad Paray, B.; Gulnaz, A.; Vladulescu, C.; Mansoor, S. Enhancement of nutraceutical and anti-diabetic potential of fenugreek (Trigonella foenum-graecum). Sprouts with natural elicitors. Saudi Pharm. J. 2023, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Choi, D.-S.; Nguyen, T.K.L.; Oh, M.-M. Growth and biochemical responses of kale to supplementary irradiation with different peak wavelengths of UV-A light-emitting diodes. Hortic. Environ. Biotechnol. 2022, 63, 65–76. [Google Scholar] [CrossRef]
- Qian, M.; Kalbina, I.; Rosenqvist, E.; Jansen, M.A.K.; Strid, Å. Supplementary UV-A and UV-B radiation differentially regulate morphology in ocimum basilicum. Photochem. Photobiol. Sci. 2023, 22, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Galvão, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Zhang, X.; Su, N.; Jia, L.; Tian, J.; Li, H.; Huang, L.; Shen, Z.; Cui, J. Transcriptome analysis of radish sprouts hypocotyls reveals the regulatory role of hydrogen-rich water in anthocyanin biosynthesis under UV-A. BMC Plant Biol. 2018, 18, 227. [Google Scholar] [CrossRef]
- Nguyen, L.T.K.; Oh, M.M. Growth and biochemical responses of green and red perilla supplementally subjected to UV-A and deep-blue LED lights. Photochem. Photobiol. 2022, 98, 1332–1342. [Google Scholar] [CrossRef]
- Johnson, G.A.; Day, T.A. Enhancement of photosynthesis in sorghum bicolor by ultraviolet radiation. Physiol. Plant. 2010, 116, 554–562. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; Ric de Vos, C.H.; Maas, F.M.; Jonker, H.H.; Van Den Broeck, H.C.; Jordi, W.; Pot, C.S.; Keizer, L.C.P.; Schapendonk, A.H.C.M. Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol. Plant. 2013, 117, 171–178. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in Pak-choi. J. Sci. Food Agric. 2020, 101, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Yamaguchi, F.; Ando, Y. Physiological activation in radish plants by UV-A radiation. J. Photochem. Photobiol. B Biol. 1994, 24, 33–40. [Google Scholar] [CrossRef]
- Xu, M.-J.; Dong, J.-F.; Zhu, M.-Y. Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. J. Sci. Food Agric. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- Jia, L.; Tian, J.; Wei, S.; Zhang, X.; Xu, X.; Shen, Z.; Shen, W.; Cui, J. Hydrogen gas mediates ascorbic acid accumulation and antioxidant system enhancement in soybean sprouts under UV-A irradiation. Sci. Rep. 2017, 7, 16366. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Ge, W.; Zhou, X.; Zhou, Q.; Wei, B.; Cheng, S.; Ji, S. Phenylpropanoid metabolism in relation to peel browning development of cold-stored ‘Nanguo’ pears. Plant Sci. 2022, 322, 111363. [Google Scholar] [CrossRef] [PubMed]
- Hideg, É.; Jansen, M.A.K.; Strid, K. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Zhuang, L.; Huang, G.; Li, X.; Xiao, J.; Guo, L. Effect of different LED lights on aliphatic glucosinolates metabolism and biochemical characteristics in broccoli sprouts. Food Res. Int. 2022, 154, 111015. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Ou, S.; Gao, M.; Zhang, Y.; Song, S.; Liu, H. Regulation of growth and main health-promoting compounds of Chinese kale baby-leaf by UV-A and FR light. Front. Plant Sci. 2022, 12, 799376. [Google Scholar] [CrossRef]
- Xie, C.; Tang, J.; Xiao, J.; Geng, X.; Guo, L. Purple light-emitting diode (LED) lights controls chlorophyll degradation and enhances nutraceutical quality of postharvest broccoli florets. Sci. Hortic. 2022, 294, 110768. [Google Scholar] [CrossRef]
- Bharadwaj, R.P.; Raju, N.G.; Chandrashekharaiah, K.S. Purification and characterization of alpha-amylase inhibitor from the seeds of underutilized legume, mucuna pruriens. J. Food Biochem. 2018, 72, 2267–2274. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J. Funct. Foods 2014, 9, 70–77. [Google Scholar] [CrossRef]
- Jia, L.; Wang, T.; Sun, Y.; Zhang, M.; Tian, J.; Chen, H.; Shen, Z.; Abro, H.; Su, N.; Cui, J. Protective effect of selenium-enriched red radish sprouts on carbon tetrachloride-induced liver injury in mice. J. Food Sci. 2019, 84, 3027–3036. [Google Scholar] [CrossRef]
- Su, N.; Liu, Z.; Wang, L.; Liu, Y.; Niu, M.; Chen, X.; Cui, J. Improving the anthocyanin accumulation of hypocotyls in radish sprouts by hemin-induced NO. BMC Plant Biol. 2022, 22, 224. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int. J. Food Sci. Nutr. 2014, 65, 476–481. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Gu, Z. Cloning of genes related to aliphatic glucosinolate metabolism and the mechanism of sulforaphane accumulation in broccoli sprouts under jasmonic acid treatment. J. Sci. Food Agric. 2016, 96, 4329–4336. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Gu, Z. Effect of freezing methods on sulforaphane formation in broccoli sprouts. RSC Adv. 2015, 5, 32290–32297. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Liu, R.H. Microwave-assisted extraction of polysaccharides from moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Ind. Crops Prod. 2017, 100, 1–11. [Google Scholar] [CrossRef]
- Ke, S.; Wei, B.; Qiu, W.; Zhou, T.; Wang, S.; Chen, J.; Chen, J.; Zhang, H.; Jin, W.; Wang, H. Structural characterization and alpha-glucosidase inhibitory and antioxidant activities of fucoidans extracted from saccharina japonica. Chem. Biodivers. 2020, 17, e2000233. [Google Scholar] [CrossRef] [PubMed]
- Ercan, P.; El, S.N. Inhibitory effects of chickpea and Tribulus terrestris on lipase, alpha-amylase and alpha-glucosidase. Food Chem. 2016, 205, 163–169. [Google Scholar] [CrossRef] [PubMed]








| μmol/g | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light Intensity/W | Aliphatic Glucosinolate | Indole Glucosinolate | Total Glucosinolate | |||||||||
| GRA | RAE | GNA | GER | RSA | PRO | 4HGB | GB | 4MGB | NGB | |||
| BSE | 0 | 3.61 ± 0.07 e | — | 0.99 ± 0.01 e | 0.35 ± 0.02 e | — | — | 0.15 ± 0.01 d | 0.36 ± 0.03 e | 0.51 ± 0.01 d | 0.11 ± 0.01 e | 6.08 ± 0.16 e |
| 4 | 5.86 ± 0.21 d | — | 1.37 ± 0.01 d | 0.52 ± 0.01 d | — | — | 0.26 ± 0.02 c | 0.39 ± 0.02 d | 0.52 ± 0.02 c | 0.15 ± 0.02 d | 9.07 ± 0.31 d | |
| 8 | 8.31 ± 0.28 c | — | 1.73 ± 0.01 c | 0.74 ± 0.00 c | — | — | 0.27 ± 0.02 b | 0.34 ± 0.04 c | 0.53 ± 0.01 b | 0.17 ± 0.02 c | 12.09 ± 0.38 c | |
| 12 | 10.83 ± 0.19 a | — | 2.17 ± 0.02 a | 1.17 ± 0.01 a | — | — | 0.33 ± 0.01 a | 0.40 ± 0.04 b | 0.54 ± 0.01 a | 0.21 ± 0.05 a | 15.62 ± 0.33 a | |
| 16 | 9.78 ± 0.21 b | — | 1.86 ± 0.02 b | 1.05 ± 0.01 b | — | — | 0.33 ± 0.00 a | 0.42 ± 0.02 a | 0.54 ± 0.01 a | 0.18 ± 0.03 b | 14.16 ± 0.30 b | |
| RSE | 0 | — | 1.47 ± 0.03 e | 0.59 ± 0.01 e | — | 0.16 ± 0.03 e | 0.22 ± 0.02 c | 0.05 ± 0.00 c | 0.22 ± 0.04 d | 0.31 ± 0.01 b | — | 3.02 ± 0.14 e |
| 4 | — | 1.89 ± 0.04 d | 0.68 ± 0.01 d | — | 0.25 ± 0.02 d | 0.25 ± 0.04 b | 0.08 ± 0.01 b | 0.23 ± 0.04 c | 0.31 ± 0.01 b | — | 3.69 ± 0.17 d | |
| 8 | — | 2.78 ± 0.04 c | 0.75 ± 0.00 c | — | 0.33 ± 0.02 c | 0.27 ± 0.02 a | 0.08 ± 0.00 b | 0.24 ± 0.04 b | 0.31 ± 0.02 b | — | 4.76 ± 0.14 c | |
| 12 | — | 4.18 ± 0.07 a | 1.04 ± 0.03 a | — | 0.56 ± 0.04 a | 0.19 ± 0.01 d | 0.09 ± 0.00 a | 0.25 ± 0.04 a | 0.32 ± 0.01 a | — | 6.63 ± 0.20 a | |
| 16 | — | 3.92 ± 0.07 b | 0.82 ± 0.02 b | — | 0.45 ± 0.05 b | 0.19 ± 0.02 d | 0.08 ± 0.02 b | 0.24 ± 0.00 b | 0.32 ± 0.02 a | — | 6.02 ± 0.20 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, G.; Chen, M.; Li, X.; Xiao, J.; Liu, L.; Guo, L. Effect of UV-A Irradiation on Bioactive Compounds Accumulation and Hypoglycemia-Related Enzymes Activities of Broccoli and Radish Sprouts. Plants 2024, 13, 450. https://0-doi-org.brum.beds.ac.uk/10.3390/plants13030450
Che G, Chen M, Li X, Xiao J, Liu L, Guo L. Effect of UV-A Irradiation on Bioactive Compounds Accumulation and Hypoglycemia-Related Enzymes Activities of Broccoli and Radish Sprouts. Plants. 2024; 13(3):450. https://0-doi-org.brum.beds.ac.uk/10.3390/plants13030450
Chicago/Turabian StyleChe, Gongheng, Mingmei Chen, Xiaodan Li, Junxia Xiao, Liang Liu, and Liping Guo. 2024. "Effect of UV-A Irradiation on Bioactive Compounds Accumulation and Hypoglycemia-Related Enzymes Activities of Broccoli and Radish Sprouts" Plants 13, no. 3: 450. https://0-doi-org.brum.beds.ac.uk/10.3390/plants13030450