1. Introduction
The genus
Carlina L. (Asteraceae) usually comprises thistle-like plants (annuals, biennials or perennials), often with prickly foliage and daisy-like flowers (compound inflorescences of ligulate and tubular florets) with spiny phyllaries (often leaf-like) that originally are wild growing in Europe, the Mediterranean region and Asia [
1,
2].
The ornamental value of
C. vulgaris L. subsp.
vulgaris (synonym
C. sylvestris Bubani) and
C. lanata L. has been reported in Europe as early as the 1620s [
3], and
C. acualis L. subsp.
acaulis (synonym
C. alpina Jacq.) was introduced in 1759 as an ornamental of magnificent appearance in gardens of Scotland [
4]. Nowadays, plant trade over the internet [
5] of some
Carlina species is extant, mainly as seeds for home gardening or landscaping.
From an ethnobotanical point of view, the flowering heads of
Carlina spp. are often used globally as a substitute for artichoke and are cooked [
6,
7,
8]. From a medicinal viewpoint, the root of
C. acualis (and/or
C. acanthifolia All.), the so-called
Carlinae radix, has been recognized since ancient and medieval times and is to be found in renaissance botanical books, several pharmacopoeas and contemporary folk medicinal traditions around Europe [
9].
Carlinae radix has mainly been used as a diuretic, diaphoretic, stomachic and externally for the treatment of skin inflammations, as well as against toothache, for treating cholecystopathy and gastrointestinal disturbances, while the root’s essential oil is proved to have antimicrobial, antibacterial, anti-inflammatory, anti-ulcer, and antioxidant activities [
9,
10].
Due to protection status or limited extent of occurrence in several countries and to address the commercial interest for
Carlina spp. (both medicinal and ornamental), various propagation and cultivation systems (field cultivation, hydroponics, in vitro cultures) have been reported to date [
11]. These mainly include micropropagation of the stemless
C. acaulis subsp.
acaulis using immature zygotic embryos [
12]; microplant production of
C. acaulis subsp.
acaulis and
C. acanthifolia subsp.
utzka (Hacq.) Meusel and A.Kástner) from callus cultures using root and stem explants [
13]; in vitro regeneration of
C. acaulis subsp.
caulescens (Lam.) Schübl. & G.Martens using shoot tips, fragments of hypocotyls, cotyledons and roots from sterile seedlings [
14,
15,
16]; shoot multiplication [
17] and rooting [
18] of
C. acanthifolia subsp.
utzka using shoot tip and hypocotyl explants. At the same line, sexual propagation of
C. vulgaris [
19] and
C. acaulis [
11] has succeeded, and seed dormancy was studied as well [
20]. The germination of
Carlina seeds is generally reported to be very high, i.e., 80–100% for
C. corymbosa L. [
21,
22], 94% for
C. acaulis subsp.
acaulis [
22] and 100% for
C. acanthifolia [
22,
23],
C. vulgaris [
22,
24],
C. biebersteinii Bernh. ex Hornem.,
C. involucrata Poir., and
Chamaeleon macrocephalus (Moris) Sch. Bip. subsp.
macrocephalus (synonym
Carlina macrocephala Moris) [
22].
Apart from the above-mentioned widespread
Carlina taxa, this genus includes another 20 single-country endemics (69% of the total taxa in the genus) and therefore uncommon, range-restricted species and subspecies [
25,
26], such as:
C. biebersteinii subsp.
sudetica Kovanda confined to parts of the Czech Republic [
22];
C. acanthifolia nothosubsp.
lecoqii (Arènes) B. Bock confined to parts of France;
C. canariensis Pit.,
C. texedae Marrero Rodr.,
C. falcata Svent. and
C. xeranthemoides L. f. confined to the Canary Islands in Spain (Gran Canaria (the first two), La Palma and/or Tenerife, respectively));
C. salicifolia (L. f.) Cav. confined to the Canary Islands (Spain) and Madeira (Portugal);
C. frigida Boiss. and Heldr. subsp.
fiumensis (Simonk.) Meusel and A. Kástner confined to parts of Croatia;
C. ×
szaferi Jasiewicz and Pawl. confined to parts of Poland;
C. guittonneaui Dobignard confined to parts of Morocco;
C. kurdica Meusel and A. Kástner confined to north-west Iraq;
C. nebrodensis Guss. (unplaced name according to Plants of the World Online) and
C. sicula Ten. subsp.
sicula confined to Sicily, Italy;
C. oligocephala Boiss. and Kotschy subsp.
oligocephala and
C. oligocephala subsp.
pallescens (Wettst.) Meusel and A. Kástner confined to Anatolia, Turkey;
C. pygmaea Holmboe confined to parts of Cyprus;
C. barnebyana B.L. Burtt and P.H. Davis,
C. curetum Heldr. subsp.
curetum, C. diae (Rech. f.) Meusel and A. Kástner and
C. sitiensis Rech. f. confined to islands of Greece (Crete and small islets and/or Karpathos and/or Kasos islands). Among the latter,
C. diae is a rock-dwelling plant of inaccessible cliff faces and steeply sloping calcareous rocks close to the sea (0–150 m) which is confined exclusively to the islets of Dia, Dragonada and Gianisada off the north coast of Crete (
Figure 1a,b), with few isolated individuals also found at Cape Movros and in a gorge close to Toplou Monastery over Karoumpes bay in north-eastern Crete [
27].
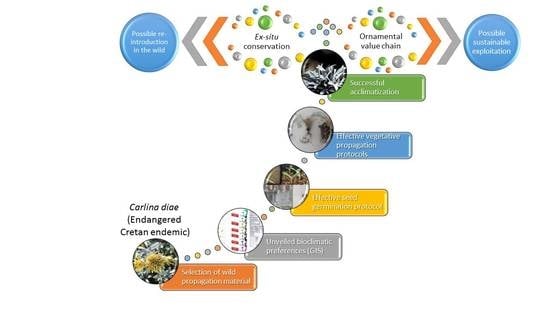
The selection of
C. diae as a focal species for this study is primarily due to the considerable scientific value associated with its uniqueness. In the first place, it is a very rare, local (Cretan) endemic plant with a total population size <1000 mature individuals remaining in the wild (severely fragmented subpopulations, each with <250 mature individuals) [
27]. All its subpopulations are included in the NATURA 2000 sites GR4310003 and GR4320006 and it is protected by the Bern Convention (Appendix I) and the Greek Presidential Decree 67/1981. However,
C. diae is threatened with extinction due to overgrazing and it is assessed as endangered with decreasing population trend [
27]. Secondly, it is considered as a Tertiary relict of the small and ancestral subgenus
Lyrolepis Meusel and Kästner., which represents “an ancient type compared to (the rest of)
Carlina spp.” [
28], thus rendering it as de facto “unusual” and therefore unique and attractive among other similar plants. It is not cultivated as a rare ornamental plant [
5] and there are only a couple of specimens conserved in ex situ facilities.
Therefore, there is need for its effective ex situ conservation [
29,
30,
31,
32]. In this context, the present study investigates the sexual and asexual reproduction of
C. diae, a potential medicinal plant with conservation priority and highly promising ornamental value, using geographical information systems (GIS) to unveil its seasonal bioclimatic preferences. This aim to present a consolidated multiplication process serving ex situ conservation efforts and facilitating sustainable exploitation needs.
3. Discussion
The development of propagation protocols for rare and threatened species is imperative for effective ex situ conservation [
34,
35,
36,
37]. The current study focused on the GIS-facilitated propagation of the rare and threatened (endangered) local Cretan endemic
C. diae by seeds in in vivo and in vitro conditions, as well as by cuttings. The results presented herein demonstrate the development of efficient propagation protocols regarding
C. diae, serving either conservation purposes or its sustainable exploitation as a new flower crop in the horticultural/ornamental industry and as a potential medicinal plant for the pharmaceutical sector. Both vegetative and sexual reproduction methods were successfully applied in
C. diae and the optimum conditions for both systems have been determined concisely. It is suggested that consideration must be given to the environment-specific needs of the plant species under propagation and conservation study [
38]. In the absence of published species-specific studies, the GIS application used in our study has offered the potential to unveil the ecological preferences of
C. diae in a meaningful way for ex situ conservation and horticulture. This helped to improve the common routine exercised in botanical gardens when dealing with new species (trial-and-error losses of plant material). In the case studied here, valuable and rare plant material was used which was acquired only in limited quantities in order to minimize possible risks for the endangered wild population. The GIS application used has contributed to the selection of: (a) appropriate temperatures for greenhouse seed germination, rooting of cuttings and cultivation, (b) appropriate period for acclimatization and transplanting of plantlets produced in vitro, and (c) spatial positioning within the nurseries and the ex situ conservation facilities of the BBGK. Our study illustrates how GIS may facilitate the seed germination, rooting of cuttings and the in vitro propagation of limited plant material of conservation priority species (such as
C. diae), and in addition provides ecologically based guidelines for its ex situ cultivation.
The seeds of
C. diae do not appear to have dormancy restrictions, as they germinated at a satisfactory rate under various conditions examined (both in vivo and in vitro) (
Table 1 and
Table 2). Although the germination capacity of
C. diae seeds was higher than 90% in both in vivo and in vitro systems, the cold storage of
C. diae seeds for 80 days enhanced the germination rate in vivo (
Table 1) and the use of GA
3 accelerated the in vitro germination process (
Table 2). This is quite common for some species in the Asteraceae family, where the efficiency of seed germination and seedling growth is low because they are highly dependent on various biological and environmental factors [
39]; therefore, treatments with plant growth regulators (PGRs) such as GA
3 promote their germination [
40]. The maximum in vivo germination rates of
C. diae seeds were achieved 30 days after sowing for both trials, although the experiments were evaluated for two months. Therefore, no sign of dormancy was observed for
C. diae, unlike many other native species in the Asteraceae family, which need special treatments to break the seed dormancy [
41]. Our results are in accordance with those of Fournaraki (2010) [
42], in which
C. diae seeds germinated well under laboratory conditions in all temperatures tested (10, 15 and 20 °C) in white light/dark (12 h/12 h) and at constant darkness (24 h), with germination delayed at the lowest temperature (10 °C) and all germination rates high (100%) at all temperatures. The species’ seeds are not characterized by primary normal dormancy and exhibit orthodox storage behavior [
42]. In line with that, the germination data in Petri dishes containing 1% agar of nine
Carlina species [
22] show high germination success (80–100%) in different photoperiod regimes (8 h light/16 h darkness or 12 h light/12 h or 24 h darkness) within a short period of 7 to 42 days and within a wide range of temperatures (5–25 °C).
This study describes for the first time a complete production process regarding
C. diae in vitro plants, from seed germination to acclimatization. Disinfection of
C. diae seeds provided a high percentage (90–98%) of non-infected seeds which were used as initial plant material for micropropagation. Although contamination is a limiting factor in general for micropropagation [
43], the disinfection of seeds used as explants in different
Carlina spp. is a common process [
11,
15,
16]. The initiation of the in vitro germination was accelerated, and it was achieved as early as on day 4 using an MS medium with GA
3 with maximum germination of 96% after 10 days, in contrast to 100% germination in a 30-day period on GA-free medium. The results are similar to those regarding
C. acaulis where the tested achenes with viable seeds germinate very well (94%) on MS medium supplemented with 2.9 μM GA
3 after 10 days of culture in continuous light (26 ± 1 °C) [
15] as well as to those concerning
C. acanthifolia subsp.
utzka [
11,
17].
Some phenotypic effects produced by light in plants are induced by PGRs and especially gibberellins (GAs). It is reported that processes such as germination, de-etiolation, root/tuber formation or stem growth are complex outcomes of interactions between light and gibberellins [
44]. Compared to the in vitro seedlings produced on plain MS in our study, the in vitro seedlings of
C. diae derived from an MS medium supplemented with 750 μM GA
3 showed a healthier appearance, larger internodes and generally more robust and vivid habit and, therefore, a better quality (
Figure 2d,e). At the same line, it is reported that
C. acaulis plantlets grown under a 16h photoperiod and treated with 100 μΜ GA
3 may develop maximum stem length increase due to the increased number and length of internodes [
12]. Such a trend may be attributed to GA
3, which is involved in stem elongation, triggered by increased photoperiod and thus a shortening of darkness [
45].
In the proliferation phase of
C. diae, the use of BA in combination with GA
3 resulted in hyperhydricity and complete destruction of cultures of
C. diae after five subcultures compared to the MS medium with GA
3 alone, which allowed a satisfactory rate of microshoot production. This trend was also observed during shoot proliferation of
C. acanthifolia subsp.
utzka [
17]. Moreover, inhibition of shoot elongation by BA has been also reported in
C. acaulis [
15,
16] and other Asteraceae species [
46,
47]. However, the use of BA has been reported in other species of Asteraceae [
48,
49,
50], as it may overcome apical dominance, promoting axillary bud development [
12,
51,
52]. In vitro culture of
C. acaulis subsp.
caulescens may be achieved with a satisfactory bud multiplication in an MS medium supplemented with 4.44 μM BA and 1.14 μΜ IAA [
12]. The best morphogenetic response of
C. acaulis can be observed when shoot tips and other explants are cultured in MS containing 13.32 μM BA and 0.54 μM NAA producing a 6.1 multiplication rate during the first five subcultures [
16]. In the current study, the
C. diae nodal explants cultured in MS medium with 2.9 μM GA
3 (BA-free) generated the highest shoot proliferation rate, achieving a 5.38-fold increase in a six-month period.
Exogenous auxins are commonly used to improve natural rooting efficiency of stem cuttings, but it has been demonstrated in various plant species that relatively high auxin concentrations are required only during the induction phase, while PGRs during development have a rather negative effect [
53]. In this study, the positive response of
C. diae cuttings (an increase in rooting rate from 70 to 100%) with the application of 1000 or 2000 ppm IBA could be due to the low supplement of endogenous auxins in the shoots of the plant and to the fact that auxin might have interacted positively with the application of exogenous rooting hormone [
54]. Similarly, the promoting effect of IBA (500 ppm) on the rooting ability of another species of the Asteraceae (i.e.,
Stevia rebaudiana (Bertoni) Bertoni) has been reported [
55]. Such a trend could either be due to the positive effect on root initiation, the formation of more and uniform roots [
54] or due to acceleration of nutrient translocation from the upper part of the cuttings to their basal ends by increasing the activity of energy-producing enzymes [
56].
A previous study related to the effect of IBA and NAA on rooting of
Chrysanthemum × morifolium (Ramat.) Hemsl. (also, Asteraceae) terminal cuttings showed that cuttings treated with 400 ppm IBA are associated with higher rooting percentage [
57]. IBA is the most extensively used auxin to enhance root induction in cuttings due to its high ability to stimulate rhizogenesis, weak toxicity and great stability with respect to NAA [
58]. The percentage of cuttings with root formation of
C. diae after application with either 1000 or 2000 ppm IBA was substantially higher than that obtained in the untreated cuttings, demonstrating the importance of synthetic auxin for asexually produced
C. diae plants. However, in our study the highest applied IBA concentration of 4000 ppm negatively affected rooting by decreasing the percentage and many side-effects were presented. It is reported that IBA might be toxic to certain softwood cuttings taken from perennial plant species, resulting in poor or absent growth and/or mortality of the cuttings due to an antagonism between the high concentration of exogenous IBA and the endogenous auxin of the plant [
59]. A similar explanation suggests that high concentrations of exogenous auxin levels in the cuttings might disturb the hormonal metabolism, exerting an inhibitory effect on root formation [
60].
In the present study, the maximum number of roots per rooted cutting (16–17) was recorded under 2000 and 4000 ppm IBA. This finding suggests that the treated cuttings with auxin at appropriate concentrations induced early and better root initiation (i.e., more roots per cutting) [
53]. The application of IBA may indirectly favor rooting by raising the translocation speed and sugar movement from the apex towards the base of the cutting [
61]. The good rooting ability of basal cuttings could be due to higher sugar reserves or due to the accumulation of natural auxins in the shoot bases or other segments promoting relatively low levels of rooting inhibitors [
62,
63], or may be due to juvenility factors [
64] found along the plant’s stem [
65]. The positive effect of IBA was also confirmed in the case of ex vitro rooting of
C. diae microshoots. This treatment allowed faster production of plants for
C. diae, by omitting the rooting stage in vitro and offered benefits regarding the hardening of microplants during the rooting process in the mist chamber in vivo. Therefore, this technique may be also recommended for the mass propagation of other similar perennial native plants.
With efficient ex situ conservation of
C. diae and successful propagation protocols that can be applied at a commercial scale, its sustainable exploitation may be addressed.
C. diae has noteworthy ornamental features which are usually appreciated by the horticultural industry: the wild-growing individuals are densely white- or silver-felted dwarf and rigid shrubs (strongly wooded below), with numerous, short, non-flowering densely crowded branches, and sparsely leafy, erect, flowering stems 40–60 cm high that are sparingly branched above. Each flowering stem has a small flat-topped cluster of one to four flower heads (
Figure 1d) of tubular dull yellow disc florets surrounded by several rows of bracts (the outer small, lanceolate, entire or with a few small lobes; the inner bright yellow, shining, scarious, and radiating) [
27,
66]. Flowering from June to August, it has potential as an attractive rock garden plant blooming throughout summertime [
66]. It may also prove to be suitable for xeriscaping due to its obligatory rock-dwelling habit and concomitant limited needs for nutrients and water, thus rendering it advantageous for gardening in arid areas with low rainfall and scarcity of water (see
Appendix A). The proximity of its wild populations to sea level (coastal chasmophyte, though not halophyte) suggests that moderate salt spraying may be tolerated, thus rendering
C. diae as suitable for gardening in coastal areas. Moreover, it could possibly be introduced as an interior pot plant, being able to withstand the low humidity indoors during winter. Furthermore, there are reports of free hybridization with
C. vulgaris in old successful glasshouse cultivation in Germany (a completely different thistle-like plant) [
1], and thus shows promise for the breeding of new ornamentals.