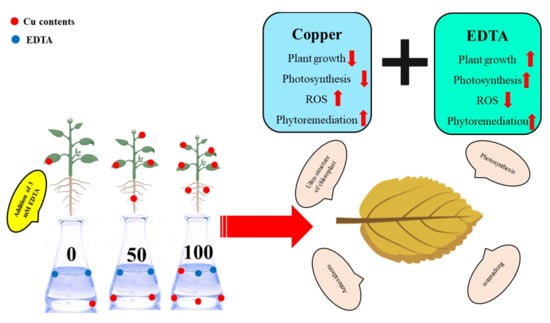
Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Treatments
2.2. Sampling and Data Collection
2.3. Determination of Chlorophyll Contents and Gaseous Exchange Parameters
2.4. Determination of Contents of Malondialdehyde and Proline and Activities of Antioxidant Enzyme
2.5. Cu Determination
2.6. Transmission Electron Microscopy
2.7. Statistical Analysis
3. Results
3.1. Plant Growth and Biomass
3.2. Chlorophyll Contents and Gaseous Exchange Attributes
3.3. Oxidative Stress and Antioxidant Enzyme Activities
3.4. Uptake and Distribution of Cu
3.5. Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al Naggar, Y.; Khalil, M.S.; Ghorab, M.A. Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc. J. Toxicol. 2018, 3, 555603. [Google Scholar]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Hashem, I.A.; Abbas, A.Y.; Abd El-Hamed, A.E.-N.H.; Salem, H.M.S.; El-hosseiny, O.E.M.; Abdel-Salam, M.A.; Saleem, M.H.; Zhou, W.; Hu, R. Potential of rice straw biochar, sulfur and ryegrass (Lolium perenne L.) in remediating soil contaminated with nickel through irrigation with untreated wastewater. PeerJ 2020, 8, e9267. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2019, 38, 266–281. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE 2018, 13, e0203612. [Google Scholar] [CrossRef]
- Wang, R.; Shafi, M.; Ma, J.; Zhong, B.; Guo, J.; Hu, X.; Xu, W.; Yang, Y.; Ruan, Z.; Wang, Y. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants. Environ. Sci. Pollut. Res. 2018, 25, 28695–28704. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, K.; Gill, R.A.; Islam, F.; Farooq, M.A.; Wang, J.; Zhou, W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napus L. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.; Maqbool, Z.; Peng, D.; Liu, L. Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ. Sci. Pollut. Res. 2019, 26, 5851–5861. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Chandrasekhar, C.; Ray, J.G. Copper accumulation, localization and antioxidant response in Eclipta alba L. in relation to quantitative variation of the metal in soil. Acta Physiol. Plant. 2017, 39, 205. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Wang, N.; Hu, S.; Wu, D.; Wang, Y. Effects of CA and EDTA on growth of Chlorophytum comosum in copper-contaminated soil. Shengtai Xuebao/Acta Ecol. Sin. 2013, 33, 631–639. [Google Scholar]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.; Sahoo, L.; Sanjib, P. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Peng, D.; Shafi, M.; Li, S.; Wu, J.; Ye, Z.; Wang, Y.; Yan, W.; Liu, D. Phytoremediation potential of moso bamboo (Phyllostachys pubescens) for zinc and ultrastructure changes under zinc toxicity. Russ. J. Ecol. 2015, 46, 444–449. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Iqbal, N.; Noman, A.; Ali, M. Influence of melatonin on antioxidant defense system and yield of wheat (Triticum aestivum L.) genotypes under saline condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Mwamba, T.M.; Gill, R.A.; Yang, C.; Ali, S.; Daud, M.K.; Wu, Y.; Zhou, W. Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul. 2014, 74, 261–273. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Niaz, M.; Kausar, A.; Hussain, J. Evaluation of wheat genotypes for salinity tolerance using physiological indices as screening tool. Pak. J. Bot. 2015, 47, 397–405. [Google Scholar]
- Habiba, U.; Ali, S.; Farid, M.; Shakoor, M.B.; Rizwan, M.; Ibrahim, M.; Abbasi, G.H.; Hayat, T.; Ali, B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Hu, C.X.; Shaaban, M.; Imran, M.; Afzal, J.; Moussa, M.G.; Elyamine, A.M.; Bhantana, P.; Saleem, M.H.; Syaifudin, M. Soil phosphorus transformation characteristics in response to molybdenum supply in leguminous crops. J. Environ. Manag. 2020, 268, 110610. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in physiological and biochemical processes in wheat supplied with zinc and potassium under saline condition. J. Plant Nutr. 2018, 41, 19–28. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, S.; Rehman, M.; Rana, M.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Hussein, M.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Bhantana, P.; Sun, X.-C.; Imran, M.; Shaaban, M.; Moussa, M.; Hamzah Saleem, M.; Elyamine, A.; Binyamin, R.; Alam, M.; et al. Molybdenum as an Essential Element for Crops: An Overview. Int. J. Sci. Res. Growth 2020, 24, 18535. [Google Scholar]
- Saleem, M.H.; Rehman, M.; Zahid, M.; Imran, M.; Xiang, W.; Liu, L. Morphological changes and antioxidative capacity of jute (Corchorus capsularis, Malvaceae) under different color light-emitting diodes. Braz. J. Bot. 2019, 42, 581–590. [Google Scholar] [CrossRef]
- Vishnoi, S.R.; Srivastava, P. Phytoremediation–Green for Environmental Clean. In Proceedings of the Taal2007: The 12th World Lake Conference, Jaipur, India, 28 October–2 November 2007; p. 1021. [Google Scholar]
- Zhang, B.; Zheng, J.; Sharp, R. Phytoremediation in engineered wetlands: Mechanisms and applications. Procedia Environ. Sci. 2010, 2, 1315–1325. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewska, A.; Hanus-Fajerska, E.; MUSZYŃSKA, E.; Ciarkowska, K. Natural organic amendments for improved phytoremediation of polluted soils: A review of recent progress. Pedosphere 2016, 26, 1–12. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; De La Pena, T.C.; Pueyo, J.J.; Talebi, M. Isolation and Characterization of Pb-Solubilizing Bacteria and Their Effects on Pb Uptake by Brassica juncea: Implications for Microbe-Assisted Phytoremediation. J. Microbiol. Biotechnol. 2018, 28, 1156–1167. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Heavy metals and metalloids as micronutrients for plants and animals. In Heavy Metals in Soils; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–209. [Google Scholar]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Ae, N. Potential for phytoextraction of copper, lead, and zinc by rice (Oryza sativa L.), soybean (Glycine max [L.] Merr.), and maize (Zea mays L.). J. Hazard. Mater. 2009, 162, 1185–1192. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Vrbová, M.; Kotrba, P.; Horáček, J.; Smýkal, P.; Švábová, L.; Větrovcová, M.; Smýkalová, I.; Griga, M. Enhanced accumulation of cadmium in Linum usitatissimum L. plants due to overproduction of metallothionein α-domain as a fusion to β-glucuronidase protein. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 112, 321–330. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Guha, G.; Gupta, K.; Chattopadhyay, D.; Mukhopadhyay, A.; Ghosh, U.C. Trend of arsenic pollution and subsequent bioaccumulation in Oryza sativa and Corchorus capsularis in Bengal Delta. Int. Lett. Nat. Sci. 2014, 16. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Uddin Nizam, M.; Mokhlesur Rahman, M.; Kim, J.-E. Phytoremediation Potential of Kenaf (Hibiscus cannabinus L.), Mesta (Hibiscus sabdariffa L.), and Jute (Corchorus capsularis L.) in Arsenic-contaminated Soil. Korean J. Environ. Agric. 2016, 35, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Niazy Abdou, M.; Wahdan, M. Citric Acid-Enhanced Phytoremediation of Lead Using Corchorus capsularis, L, and Eucalyptus camaldulensis; ResearchGate: Berlin, Germany, 2017. [Google Scholar]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, M. Effect of EDTA and DTPA on phytoremediation of Pb-Zn contaminated soils by Eucalyptus camaldulensis Dehnh and Effect on Treatment Time. Desert 2014, 19, 65–73. [Google Scholar]
- Lambrechts, T.; Gustot, Q.; Couder, E.; Houben, D.; Iserentant, A.; Lutts, S. Comparison of EDTA-enhanced phytoextraction and phytostabilisation strategies with Lolium perenne on a heavy metal contaminated soil. Chemosphere 2011, 85, 1290–1298. [Google Scholar] [CrossRef]
- Prieto, C.; Lozano, J.; Rodríguez, P.B.; Tomé, F.V. Enhancing radium solubilization in soils by citrate, EDTA, and EDDS chelating amendments. J. Hazard. Mater. 2013, 250, 439–446. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.-M.; Huang, X.-F.; Xia, B.; Su, C.-Y.; Luo, G.-F.; Xu, Y.-W.; Wu, Y.-X.; Mao, Z.-W.; Qiu, R.-L. Chelant extraction of heavy metals from contaminated soils using new selective EDTA derivatives. J. Hazard. Mater. 2013, 262, 464–471. [Google Scholar] [CrossRef]
- Qiao, J.; Sun, H.; Luo, X.; Zhang, W.; Mathews, S.; Yin, X. EDTA-assisted leaching of Pb and Cd from contaminated soil. Chemosphere 2017, 167, 422–428. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, S.; Lin, H.; Li, T.; Xu, X.; Li, Y.; Jia, Y.; Gong, G. Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 2013, 91, 1362–1367. [Google Scholar] [CrossRef]
- Muhammad, D.; Chen, F.; Zhao, J.; Zhang, G.; Wu, F. Comparison of EDTA-and citric acid-enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia. Int. J. Phytoremediat. 2009, 11, 558–574. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Q.; Qi, Y.; Duo, L. Responses of root growth and protective enzymes to copper stress in turfgrass. Acta Biol. Crac. Ser. Bot. 2010, 52, 7–11. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Shakoor, M.B.; Bharwana, S.A.; Rizvi, H.; Ehsan, S.; Tauqeer, H.M.; Iftikhar, U.; Hannan, F. EDTA assisted phytoremediation of cadmium, lead and zinc. Int. J. Agron. Plant Prod. 2013, 4, 2833–2846. [Google Scholar]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Khan, M.H.U.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Irshad, S.; Hussaan, M.; Rizwan, M.; Rana, M.S.; Hashem, A.; Abd_Allah, E.F.; Ahmad, P. Copper Uptake and Accumulation, Ultra-Structural Alteration, and Bast Fibre Yield and Quality of Fibrous Jute (Corchorus capsularis L.) Plants Grown Under Two Different Soils of China. Plants 2020, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Mohamed, I.A.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R.; Liu, L. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 2020, 2020, 100895. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rehman, M.; Kamran, M.; Afzal, J.; Noushahi, H.A.; Liu, L. Investigating the potential of different jute varieties for phytoremediation of copper-contaminated soil. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Chigbo, C.; Batty, L. Effect of EDTA and citric acid on phytoremediation of Cr-B [a] P-co-contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 8955–8963. [Google Scholar] [CrossRef]
- Azhar, N.; Ashraf, M.Y.; Hussain, M.; Hussain, F. Phytoextraction of lead (Pb) by EDTA application through sunflower (Helianthus annuus L.) cultivation: Seedling growth studies. Pak. J. Bot. 2006, 38, 1551–1560. [Google Scholar]
- Yang, X.; Long, X.; Ye, H.; He, Z.; Calvert, D.; Stoffella, P. Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, R.B. Prospects for Genetically Increasing the Photosynthetic Capacity of Crops. In Perspectives in Biochemical and Genetic Regulation of Photosynthesis; Alan R. Liss Inc.: New York, NY, USA, 1990. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sakharov, I.Y.; Ardila, G.B. Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]
- Wisniewski, L.; Dickinson, N.M. Toxicity of copper to Quercus robur (English Oak) seedlings from a copper-rich soil. Environ. Exp. Bot. 2003, 50, 99–107. [Google Scholar] [CrossRef]
- Alaoui-Sossé, B.; Genet, P.; Vinit-Dunand, F.; Toussaint, M.-L.; Epron, D.; Badot, P.-M. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004, 166, 1213–1218. [Google Scholar] [CrossRef]
- Lombardi, L.; Sebastiani, L. Copper toxicity in Prunuscerasifera: Growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci. 2005, 168, 797–802. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Yang, W.-D.; Wang, Y.-Y.; Zhao, F.-L.; Ding, Z.-L.; Zhang, X.-C.; Zhu, Z.-Q.; Yang, X.-E. Variation in copper and zinc tolerance and accumulation in 12 willow clones: Implications for phytoextraction. J. Zhejiang Univ.-Sci. B 2014, 15, 788–800. [Google Scholar] [CrossRef] [Green Version]
- Zvezdanović, J.; Marković, D.; Nikolić, G. Different possibilities for the formation of complexes of copper and zinc with chlorophyll inside photosynthetic organelles: Chloroplasts and thylakoids. J. Serb. Chem. Soc. 2007, 72, 1053–1062. [Google Scholar] [CrossRef]
- Zhao, Z.; Xi, M.; Jiang, G.; Liu, X.; Bai, Z.; Huang, Y. Effects of IDSA, EDDS and EDTA on heavy metals accumulation in hydroponically grown maize (Zea mays, L.). J. Hazard. Mater. 2010, 181, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Bačkor, M. Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Pollut. 2007, 185, 185–193. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.L.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Armbrust, L.C. Optimal copper supply is required for normal plant iron deficiency responses. Plant Signal. Behav. 2013, 8, e26611. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Haouari, C.C.; Nasraoui, A.H.; Bouthour, D.; Houda, M.D.; Daieb, C.B.; Mnai, J.; Gouia, H. Response of tomato (Solanum lycopersicon) to cadmium toxicity: Growth, element uptake, chlorophyll content and photosynthesis rate. Afr. J. Plant Sci. 2012, 6, 1–7. [Google Scholar]
- Ahmed, D.A.; Slima, D.F. Heavy metal accumulation by Corchorus olitorius L. irrigated with wastewater. Environ. Sci. Pollut. Res. 2018, 25, 14996–15005. [Google Scholar] [CrossRef]
- Wodala, B.; Eitel, G.; Gyula, T.; Ördög, A.; Horváth, F. Monitoring moderate Cu and Cd toxicity by chlorophyll fluorescence and P 700 absorbance in pea leaves. Photosynthetica 2012, 50, 380–386. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef]
- Ali, B.; Wang, B.; Ali, S.; Ghani, M.; Hayat, M.; Yang, C.; Xu, L.; Zhou, W. 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J. Plant Growth Regul. 2013, 32, 604–614. [Google Scholar] [CrossRef]
- Zengin, F.K.; Kirbag, S. Effects of copper on chlorophyll, proline, protein and abscisic acid level of sunflower (Helianthus annuus L.) seedlings. J. Environ. Biol. 2007, 28, 561. [Google Scholar] [PubMed]
- Mei, L.; Daud, M.; Ullah, N.; Ali, S.; Khan, M.; Malik, Z.; Zhu, S. Pretreatment with salicylic acid and ascorbic acid significantly mitigate oxidative stress induced by copper in cotton genotypes. Environ. Sci. Pollut. Res. 2015, 22, 9922–9931. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, W.; Yang, W.; Liang, L.; Yao, W.; Chai, L.; Gao, S.; Liao, Q. Combination of bioleaching by gross bacterial biosurfactants and flocculation: A potential remediation for the heavy metal contaminated soils. Chemosphere 2018, 206, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Saleem, M.H.; Moussa, M.G.; Bhantana, P.; Afzal, J. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. Int. J. Mol. Sci. 2019, 20, 3009. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Rehman, M.; Fahad, S.; Tung, S.A.; Iqbal, N.; Hassan, A.; Ayub, A.; Wahid, M.A.; Shaukat, S.; Liu, L.; et al. Leaf gas exchange, oxidative stress, and physiological attributes of rapeseed (Brassica napus L.) grown under different light-emitting diodes. Photosynthetica 2020, 58, 836–845. [Google Scholar] [CrossRef]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Yang, M.; Fahad, S.; Saleem, M.H.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological traits, antioxidant capacity and nitrogen metabolism in Boehmeria nivea L. under nitrogen fertilizer. Agron. J. 2020, 1–10. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Quartacci, M.; Ranieri, A.; Sgherri, C. Antioxidative defence mechanisms in two grapevine (Vitis vinifera L.) cultivars grown under boron excess in the irrigation water. VITIS J. Grapevine Res. 2015, 54, 51–58. [Google Scholar]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T. Effect of Citric Acid on Growth, Ecophysiology, Chloroplast Ultrastructure, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Seedlings Exposed to Copper Stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Rehman, M.; Saud, S.; Jamal, Y.; Khan, S.; Liu, L. Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. PeerJ 2020, 8, e8321. [Google Scholar] [CrossRef] [Green Version]
- Collin, B.; Doelsch, E.; Keller, C.; Panfili, F.; Meunier, J.-D. Effects of silicon and copper on bamboo grown hydroponically. Environ. Sci. Pollut. Res. 2013, 20, 6482–6495. [Google Scholar] [CrossRef]
- Tie, S.G.; Tang, Z.J.; Zhao, Y.M.; Li, W. Oxidative damage and antioxidant response caused by excess copper in leaves of maize. Afr. J. Biotechnol. 2012, 11, 4378–4384. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Suthar, V.; Memon, K.S.; Mahmood-ul-Hassan, M. EDTA-enhanced phytoremediation of contaminated calcareous soils: Heavy metal bioavailability, extractability, and uptake by maize and sesbania. Environ. Monit. Assess. 2014, 186, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Grčman, H.; Velikonja-Bolta, Š.; Vodnik, D.; Kos, B.; Leštan, D. EDTA enhanced heavy metal phytoextraction: Metal accumulation, leaching and toxicity. Plant Soil 2001, 235, 105–114. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeterior. Biodegrad. 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, I.-S. Comparison of the ability of organic acids and EDTA to enhance the phytoextraction of metals from a multi-metal contaminated soil. Bull. Environ. Contam. Toxicol. 2010, 84, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.; Bauer, U.; Ebel, M.; Schaeffer, A. The influence of EDDS and EDTA on the uptake of heavy metals of Cd and Cu from soil with tobacco Nicotiana tabacum. Chemosphere 2007, 68, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sinhal, V.; Srivastava, A.; Singh, V. EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J. Environ. Biol. 2010, 31, 255. [Google Scholar]
- Yu, X.-Z.; Gu, J.-D. The role of EDTA in phytoextraction of hexavalent and trivalent chromium by two willow trees. Ecotoxicology 2008, 17, 143–152. [Google Scholar] [CrossRef]
- Meers, E.; Qadir, M.; De Caritat, P.; Tack, F.; Du Laing, G.; Zia, M. EDTA-assisted Pb phytoextraction. Chemosphere 2009, 74, 1279–1291. [Google Scholar]





| Treatments | Plant Height | Plant Diameter | Plant Fresh Weight | Plant Dry Weight | Total Chlorophyll |
|---|---|---|---|---|---|
| Control | 23 ± 0.2 a | 2.0 1± 0.03 b | 2.43 ± 0.04 b | 1.59 ± 0.03 b | 3 ± 0.05 b |
| EDTA | 23 ± 0.3 a | 2.09 ± 0.01 a | 2.58 ± 0.02 a | 1.65 ± 0.03 a | 3.2 ± 0.09 a |
| Cu50 | 15 ± 0.1 c | 1.79 ± 0.04 d | 2.25 ± 0.04 d | 1.33 ± 0.02 d | 2 ± 0.04 d |
| Cu50 + EDTA | 16 ± 0.2 b | 1.89 ± 0.02 c | 2.35 ± 0.03 c | 1.43 ± 0.02 c | 2.1 ± 0.06 c |
| Cu100 | 11 ± 0.2 e | 1.51 ± 0.03 f | 1.89 ± 0.05 f | 1.03 ± 0.02 f | 1.4 ± 0.09 f |
| Cu100 + EDTA | 12 ± 0.3 d | 1.62 ± 0.02 e | 2.01 ± 0.05 e | 1.14 ± 0.03 e | 1.7 ± 0.03 e |
| Treatments | Cu Concentration in Roots | Cu Concentration in Shoots |
|---|---|---|
| Control | 13 ± 2.5 e | 10 ± 1.4 f |
| EDTA | 15 ± 2.5 e | 15 ± 1.4 f |
| Cu50 | 32 ± 0.5 d | 26 ± 1 d |
| Cu50 + EDTA | 37 ± 1.2 c | 33 ± 0.9 c |
| Cu100 | 49 ± 1 b | 40 ± 0.8 b |
| Cu100 + EDTA | 57 ± 1 a | 45 ± 1 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M.; et al. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9060756
Saleem MH, Ali S, Kamran M, Iqbal N, Azeem M, Tariq Javed M, Ali Q, Zulqurnain Haider M, Irshad S, Rizwan M, et al. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants. 2020; 9(6):756. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9060756
Chicago/Turabian StyleSaleem, Muhammad Hamzah, Shafaqat Ali, Muhammad Kamran, Naeem Iqbal, Muhammad Azeem, Muhammad Tariq Javed, Qasim Ali, Muhammad Zulqurnain Haider, Sana Irshad, Muhammad Rizwan, and et al. 2020. "Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities" Plants 9, no. 6: 756. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9060756