Effects of Postharvest Water Deficits on the Physiological Behavior of Early-Maturing Nectarine Trees
Abstract
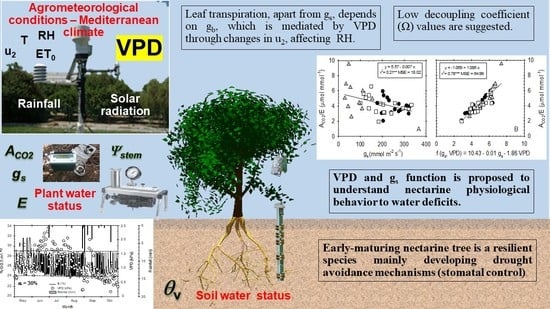
:1. Introduction
2. Results and Discussion
2.1. Water Applied and Meteorological Conditions
2.2. Time Course of Plant–Water Relations
2.3. Leaf Gas Exchange Relationships
3. Materials and Methods
3.1. Plant Material and Experimental Conditions
3.2. Irrigation Treatments
3.3. Measurements
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández-García, I.; Lecina, S.; Ruiz-Sánchez, M.C.; Vera, J.; Conejero, W.; Conesa, M.R.; Dominguez, A.; Pardo, J.J.; Léllis, B.C.; Montesinos, P. Trends and challenges in irrigation scheduling in the semi-arid area of Spain. Water 2020, 12, 785. [Google Scholar] [CrossRef] [Green Version]
- Feller, U.; Kingston-Smith, A.H.; Centritto, M. Abiotic Stresses in Agroecology: A Challenge for Whole Plant Physiology. Front. Environ. Sci. 2017, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Slattery, R.A.; Ort, D.R. Carbon assimilation in crops at high temperatures. Plant Cell Environ. 2019, 42, 2750–2758. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Abrisqueta, I.; Conejero, W.; Vera, J. Deficit irrigation management in early-maturing peach crop. In Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops; Ivan, F.G.T., Victor, H.D.Z., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 111–126. [Google Scholar]
- Conesa, M.R.; Conejero, W.; Vera, J.; Ramírez-Cuesta, J.M.; Ruiz-Sánchez, M.C. Terrestrial and remote indexes to assess moderate deficit irrigation in early-maturing nectarine trees. Agronomy 2019, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAST. Food and Agriculture Organization Statistical Data. Available online: http://www.faco.org/faostat/en/#data/QC (accessed on 22 August 2020).
- Buendía, B.; Allende, A.; Nicolás, E.; Alarcón, J.J.; Gil, M.I. Effect of Regulated Deficit Irrigation and Crop Load on the Antioxidant Compounds of Peaches. J. Agric. Food Chem. 2008, 56, 3601–3608. [Google Scholar] [CrossRef]
- Abrisqueta, I.; Tapia, L.M.; Conejero, W.; Sánchez-Toribio, M.I.; Abrisqueta, J.M.; Vera, J.; Ruiz-Sánchez, M.C. Response of early-maturing peach [Prunus persica (L.)] trees to deficit irrigation. Span. J. Agric. Res. 2010, 8, 30–39. [Google Scholar] [CrossRef]
- Alcobendas, R.; Mirás-Avalos, J.M.; Alarcón, J.J.; Pedrero, F.; Nicolás, E. Combined effects of irrigation, crop load and fruit position on size, color and firmness of fruits in an extra-early cultivar of peach. Sci. Hortic. 2012, 142, 128–135. [Google Scholar] [CrossRef]
- Vera, J.; Abrisqueta, I.; Abrisqueta, J.M.; Ruiz-Sánchez, M.C. Effect of deficit irrigation on early-maturing peach tree performance. Irrig. Sci. 2013, 31, 747–757. [Google Scholar] [CrossRef]
- Vera, J.; Conejero, W.; Conesa, M.R.; Ruiz-Sánchez, M.C. Irrigation factor approach based on soil water content: A nectarine orchard case study. Water 2019, 11, 589. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, J.M.; Domingo, R.; Gómez-Montiel, J.; Pérez-Pastor, A. Implementing deficit irrigation scheduling through plant water stress indicators in early nectarine trees. Agric. Water Manag. 2015, 152, 207–216. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Conesa, M.R.; Domingo, R.; Aguayo, E.; Falagán, E.; Pérez-Pastor, A. Combined effects of deficit irrigation and crop level on early nectarine trees. Agri. Water Manag. 2016, 170, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Sánchez, M.C.; Domingo, R.; Castel, J.R. Review. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Handley, D.F.; Johnson, R.S. Late summer irrigation of water-stressed peach trees reduces fruit doubles and deep sutures. HortScience 2000, 35, 771. [Google Scholar] [CrossRef] [Green Version]
- Dichio, B.; Xiloyannis, C.; Sofo, A.; Montanaro, G. Effects of post-harvest regulated deficit irrigation on carbohydrate and nitrogen partitioning, yield quality and vegetative growth of peach trees. Plant Soil 2007, 290, 127. [Google Scholar] [CrossRef]
- Mirá-García, A.B.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Leaf water relations in Lime trees grown under shade netting and open-air. Plants 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghrab, M.; Gargouri, K.; Bentaher, H.; Chartzoulakis, K.; Ayadi, M.; Mimoun, M.B.; Masmoudi, M.M.; Mechlia, N.B.; Psarras, G. Water relations and yield of olive tree (cv. Chemlali) in response to partial root-zone drying (PRD) irrigation technique and salinity under arid climate. Agric. Water Manag. 2013, 123, 1–11. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation-hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [Green Version]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Phyisological tolos for irrigation scheduling in grape vines (Vitis vinifera L.). Agric. Ecos. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integ. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Egea, G.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Conesa, M.R.; Ruiz-Salleres, I. Effects of water stress on irradiance acclimation of leaf traits in almond trees. Tree Physiol. 2012, 32, 450–463. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.; Pou, A.; Fuentes, S. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Sánchez, M.C.; Domingo, R.; Savé, R.; Biel, C.; Torrecillas, A. Effects of water stress and rewatering on leaf water relations of lemon plants. Biol. Plant. 1997, 39, 623–631. [Google Scholar] [CrossRef]
- Munns, R. Why measure osmotic adjustment? Aust. J. Plant Physiol. 1988, 15, 717–726. [Google Scholar] [CrossRef]
- Savé, R.J.; Peñuelas, J.O.; Marfá, O.; Serrano, L. Changes in leaf osmotic and elastic properties and canopy structure of strawberries under mild water stress. HortScience 1993, 28, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Mellisho, C.D.; Cruz, Z.N.; Conejero, W.; Ortuño, M.F.; Rodriguez, P. Mechanisms for drought resistance in early maturing var. Flordastar peach trees. J. Agric. Sci. 2011, 149, 609–616. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 2016, 6, 20917. [Google Scholar] [CrossRef] [Green Version]
- Mounzer, O.H.; Vera, J.; Tapia, L.M.; García-Orellana, Y.; Conejero, W.; Abrisqueta, I.; Ruiz-Sánchez, M.C.; Abrisqueta, J.M. Irrigation scheduling of peach trees by continuous measurement of soil water status. Agrociencia 2008, 42, 857–868. [Google Scholar]
- Abrisqueta, I.; Vera, J.; Tapia, L.; Abrisqueta, J.; Ruiz-Sánchez, M. Soil water content criteria for peach trees water stress detection during the postharvest period. Agric. Water Manag. 2012, 104, 62–67. [Google Scholar] [CrossRef]
- Abrisqueta, I.; Conejero, W.; López-Martínez, L.; Vera, J.; Ruiz-Sánchez, M.C. Root and aerial growth in early-maturing peach trees under two crop load treatments. Span. J. Agric. Res. 2017, 15, e0803. [Google Scholar] [CrossRef] [Green Version]
- Millán, S.; Casadesús, J.; Campillo, C.; Moñino, M.J.; Prieto, M.H. Using soil moisture sensors for automated irrigation scheduling in a plum crop. Water 2019, 11, 2061. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Niño, J.M.; Oliver-Manera, J.; Girona, J.; Casadesús, J. Differential irrigation scheduling by an automated algorithm of water balance tuned by capacitance-type soil moisture sensors. Agric. Water Manag. 2020, 228, 105880. [Google Scholar] [CrossRef]
- Osorio, M.L.; Breia, E.; Rodrigues, A.P.; Chaves, M.M. Limitations to carbon assimilation by mild drought in nectarine trees growing under field conditions. Environ. Exp. Bot. 2006, 55, 235–347. [Google Scholar] [CrossRef]
- Gates, D.M. Transpiration and Leaf temperature. Annu. Rev. Plant. Biol. 2003, 19, 211–238. [Google Scholar] [CrossRef]
- Turner, N.C.; Schulze, E.D.; Gollan, T. The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. Oecologia 1984, 63, 338–342. [Google Scholar] [CrossRef]
- Maroco, J.P.; Pereira, J.S.; Chaves, M.M. Stomatal responses to leaf-to-air vapour pressure deficit in Sahelian species. Aust. J. Plant Physiol. 1997, 24, 381–387. [Google Scholar] [CrossRef]
- Comstock, J.; Ehkeringer, J. Correlating genetic variation in carbon isotopic composition with complex climatic gradients. Proc. Natl. Acad. Sci. USA 1992, 89, 7745–7751. [Google Scholar] [CrossRef] [Green Version]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.M.; Tsimplis, M.; et al. The Mediterranean climate: An overview of the main characteristics and issues. In Mediterranean Climate Variability; Lionello, P., Malanotte-Rizzoli, P., Boscolo, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 1–26. [Google Scholar]
- Conesa, M.R.; Martínez-López, L.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Summer pruning of early-maturing Prunus persica: Water implications. Sci. Hort. 2019, 256. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Conesa, M.R.; Domingo, R.; Torres, R.; Pérez-Pastor, A. Feasibility of using trunk diameter fluctuation and stem water potential reference lines for irrigation scheduling of early nectarine trees. Agric. Water Manag. 2013, 126, 133–141. [Google Scholar] [CrossRef]
- Naor, A. Irrigation scheduling and evaluation of tree water status in deciduous orchards. Hortic. Rev. 2010, 32, 111–165. [Google Scholar]
- Abrisqueta, I.; Conejero, W.; Valdés-Vela, M.; Vera, J.; Ortuño, M.F.; Ruiz-Sánchez, M.C.; Ortuño, M.F. Stem water potential estimation of drip-irrigated early-maturing peach trees under Mediterranean conditions. Comput. Electron. Agric. 2015, 114, 7–13. [Google Scholar] [CrossRef]
- De Souza, C.R.; Maroco, J.P.; Santos, T.P.; Rodrigues, M.L.; Lopes, C.; Pereira, L.S.; Chaves, M.M. Control of stomatal aperture and carbon uptake by deficit irrigation in two grapevine cultivars. Agric. Ecosyst. Environ. 2005, 106, 261–274. [Google Scholar] [CrossRef]
- Rahmati, M.; Davarynejad, G.H.; Génard, M.; Bannayan, M.; Azizi, M.; Vercambre, G. Peach water relations, gas exchange, growth and shoot mortality under water deficit in semi-arid weather conditions. PLoS ONE 2015, 10, e0120246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Sánchez, M.C.; Domingo, R.; Torrecillas, A.; Pérez-Pastor, A. Water stress preconditioning to improve drought resistance in young apricot plants. Plant Sci. 2000, 156, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Conesa, M.; Dodd, I.; Temnani, A.; De La Rosa, J.; Pérez-Pastor, A. Physiological response of post-veraison deficit irrigation strategies and growth patterns of table grapes (cv. Crimson Seedless). Agric. Water Manag. 2018, 208, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, T.A.; Davies, W.J. Stomata and stomatal mechanisms. In The Physiology and Biochemistry of Drought Resistance in Plants; Leslie, G.P., Donald, A., Eds.; Academic Press: New York, NY, USA, 1981; pp. 315–346. [Google Scholar]
- Ben Mimoun, M.; Lescourret, F.; Génard, M. Modelling carbon allocation in peach shoot bearing fruits: Simulation of the water stress effect. Fruits 1999, 54, 129–134. [Google Scholar]
- Medrano, H.; Escalona, J.; Bota, J.; Gulias, J.; Flexas, J. Regulation of photosynthesis of C-3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Shackel, K.; Lampinen, B.; Sibbett, S.; Olson, W. The relation of midday stem water potential to the growth and physiology of fruit trees under water limited conditions. Acta Hortic. 2000, 537, 425–430. [Google Scholar] [CrossRef]
- López, G.; Mata, M.; Arbones, A.; Solans, J.R.; Girona, J.; Marsal, J. Mitigation of effects of extreme drought during stage III of peach fruit development by summer pruning and fruit thinning. Tree Physiol. 2006, 26, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Romero, P.; Fernández-Fernández, J.; Martínez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar]
- Flexas, J.; Niinemets, U.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Galle, A.; Florez-Sarasa, I.; Tomas, M.; Pou, A.; Medrano, H.; Ribas-Carbo, M.; Flexas, J. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): Acclimation or limitation? J. Exp. Bot. 2009, 60, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Galle, A.; Florez-Sarasa, I.; Aououad, H.E.; Flexas, J. The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J. Exp. Bot. 2011, 62, 5207–5216. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Medrano, H.; Bota, J.; Cifre, J.; Flexas, J.; Ribas-Carbó, M.; Gulías, J. Eficiencia en el uso del agua por las plantas. Investig. Geogr. 2007, 43, 63–84. [Google Scholar] [CrossRef] [Green Version]
- Bierhuizen, J.F.; Slatyer, R.O. Effect of atmospheric concentration of water vapour and CO2 in determining transpiration-photosynthesis relationships of cotton leaves. Agric. Meterol. 1965, 2, 259–270. [Google Scholar] [CrossRef]
- Martin, T.A.; Hinckley, T.M.; Meinzer, F.C.; Sprugel, D.G. Boundary layer conductance, leaf temperature and transpiration of Abies amabilis branches. Tree Physiol. 1999, 19, 435–443. [Google Scholar] [CrossRef]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 2nd ed.; Butterworth-Heinemann Elsevier: Oxford, UK, 1990; p. 414. [Google Scholar]
- Nicolás, E.; Barradas, V.L.; Ortuño, M.F.; Navarro, A.; Torrecillas, A.; Alarcón, J.J. Environmental and stomatal control of transpiration, canopy conductance and decoupling coefficient in young lemon trees under shading net. Environ. Exp. Bot. 2008, 63, 200–206. [Google Scholar] [CrossRef]
- Vera, J.; de la Peña, J.M. FERTIGA: Programa de Fertirrigación de Frutales; CEBAS-CSIC: Murcia, Spain, 1994; p. 69. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Abrisqueta, I.; Abrisqueta, J.; Tapia, L.; Munguía, J.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Basal crop coefficients for early-season peach trees. Agric. Water Manag. 2013, 121, 158–163. [Google Scholar] [CrossRef]
- Méndez, A.; Blaya, J.M.; López-Torres, F.J.; Rodríguez, E.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Distribución de Raíces de Nectarino en Distintas Condiciones de Riego; II Congreso IDIES: Murcia, Spain, 2015. [Google Scholar]
- Hsiao, T.C. Measurement of tree water status. In Irrigation of Agricultural Crops; Steward, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 243–279. [Google Scholar]
- McCutchan, H.; Shackel, K. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef] [Green Version]

 ). Each point is the mean ± standard error (n = 4). Asterisks indicate statistically significant differences between T–C and T–M (black) and T–S (gray) according to LSD0.05.
). Each point is the mean ± standard error (n = 4). Asterisks indicate statistically significant differences between T–C and T–M (black) and T–S (gray) according to LSD0.05.
 ). Each point is the mean ± standard error (n = 4). Asterisks indicate statistically significant differences between T–C and T–M (black) and T–S (gray) according to LSD0.05.
). Each point is the mean ± standard error (n = 4). Asterisks indicate statistically significant differences between T–C and T–M (black) and T–S (gray) according to LSD0.05.
 ). Each point is the mean of four leaves. Coefficient of determination for T–C (r2 = 0.71***), T–M (r2 = 0.83***), and T–S (r2 = 0.96***). ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of four leaves. Coefficient of determination for T–C (r2 = 0.71***), T–M (r2 = 0.83***), and T–S (r2 = 0.96***). ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of four leaves. Coefficient of determination for T–C (r2 = 0.71***), T–M (r2 = 0.83***), and T–S (r2 = 0.96***). ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of four leaves. Coefficient of determination for T–C (r2 = 0.71***), T–M (r2 = 0.83***), and T–S (r2 = 0.96***). ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of four leaves. Coefficient of determination in (A): T–C (r2 = 0.002 ns), T–M (r2 = 0.015 ns), and T–S (r2 = 0.52***); and in (B): T–C (r2 = 0.25***), T–M (r2 = 0.50***), and T–S (r2 = 0.70***). ns: not significant, ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of four leaves. Coefficient of determination in (A): T–C (r2 = 0.002 ns), T–M (r2 = 0.015 ns), and T–S (r2 = 0.52***); and in (B): T–C (r2 = 0.25***), T–M (r2 = 0.50***), and T–S (r2 = 0.70***). ns: not significant, ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of four leaves. Coefficient of determination in (A): T–C (r2 = 0.002 ns), T–M (r2 = 0.015 ns), and T–S (r2 = 0.52***); and in (B): T–C (r2 = 0.25***), T–M (r2 = 0.50***), and T–S (r2 = 0.70***). ns: not significant, ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of four leaves. Coefficient of determination in (A): T–C (r2 = 0.002 ns), T–M (r2 = 0.015 ns), and T–S (r2 = 0.52***); and in (B): T–C (r2 = 0.25***), T–M (r2 = 0.50***), and T–S (r2 = 0.70***). ns: not significant, ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.72***), T–S (r2 = 0.70***), T–S (r2 = 0.66*); and in (B): T–C (r2 = 0.15 ns), T–M (r2 = 0.10 ns), and T–S (r2 = 0.06 ns). ns: not significant, *: p ≤ 0.05, ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.72***), T–S (r2 = 0.70***), T–S (r2 = 0.66*); and in (B): T–C (r2 = 0.15 ns), T–M (r2 = 0.10 ns), and T–S (r2 = 0.06 ns). ns: not significant, *: p ≤ 0.05, ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.72***), T–S (r2 = 0.70***), T–S (r2 = 0.66*); and in (B): T–C (r2 = 0.15 ns), T–M (r2 = 0.10 ns), and T–S (r2 = 0.06 ns). ns: not significant, *: p ≤ 0.05, ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.72***), T–S (r2 = 0.70***), T–S (r2 = 0.66*); and in (B): T–C (r2 = 0.15 ns), T–M (r2 = 0.10 ns), and T–S (r2 = 0.06 ns). ns: not significant, *: p ≤ 0.05, ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.05 ns), T–S (r2 = 0.15 ns), T–S (r2 = 0. 06 ns); and in (B): T–C (r2 = 0.79***), T–M (r2 = 0.77***), and T–S (r2 = 0.85***). *: p ≤ 0.05 **: p ≤ 0.01, ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.05 ns), T–S (r2 = 0.15 ns), T–S (r2 = 0. 06 ns); and in (B): T–C (r2 = 0.79***), T–M (r2 = 0.77***), and T–S (r2 = 0.85***). *: p ≤ 0.05 **: p ≤ 0.01, ***: p ≤ 0.001, MSE: mean squared error.
 ). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.05 ns), T–S (r2 = 0.15 ns), T–S (r2 = 0. 06 ns); and in (B): T–C (r2 = 0.79***), T–M (r2 = 0.77***), and T–S (r2 = 0.85***). *: p ≤ 0.05 **: p ≤ 0.01, ***: p ≤ 0.001, MSE: mean squared error.
). Each point is the mean of 4 leaves. Coefficient of determination in (A): T–C (r2 = 0.05 ns), T–S (r2 = 0.15 ns), T–S (r2 = 0. 06 ns); and in (B): T–C (r2 = 0.79***), T–M (r2 = 0.77***), and T–S (r2 = 0.85***). *: p ≤ 0.05 **: p ≤ 0.01, ***: p ≤ 0.001, MSE: mean squared error.

| ET0 (mm) | Temperature (°C) | RH (%) | VPD (kPa) | Rainfall (mm) | u2 (km d−1) | Solar Radiation (W m−2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | Tmax | Tmean | Tmin | RHmax | RHmean | RHmin | |||||
| May | 140.3 | 27 | 20.7 | 14.6 | 82.7 | 57.5 | 32.3 | 1.1 | 0 | 5.42 | 243.2 |
| June | 304.8 | 31.5 | 25.3 | 19.2 | 82.5 | 56.1 | 29.6 | 1.6 | 0 | 4.38 | 275.4 |
| July | 158.7 | 32.3 | 26.6 | 21 | 88.8 | 63.1 | 37.5 | 1.5 | 0.4 | 4.88 | 248.2 |
| August | 123 | 32.9 | 27.1 | 22 | 91.2 | 64.3 | 42.1 | 1.1 | 8 | 3.2 | 218.2 |
| September | 219.5 | 30 | 24.4 | 20.2 | 92 | 70.1 | 41.1 | 0.9 | 22.6 | 4.8 | 176.4 |
| October | 71.3 | 24 | 18.8 | 14.4 | 93.7 | 68.2 | 46.3 | 0.6 | 5.2 | 3.61 | 137.4 |
| Total/Mean | 1017.6 | 29.6 | 20.5 | 18.5 | 88.5 | 63.2 | 38.2 | 1.1 | 36.2 | 4.38 | 216.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, M.R.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Effects of Postharvest Water Deficits on the Physiological Behavior of Early-Maturing Nectarine Trees. Plants 2020, 9, 1104. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9091104
Conesa MR, Conejero W, Vera J, Ruiz-Sánchez MC. Effects of Postharvest Water Deficits on the Physiological Behavior of Early-Maturing Nectarine Trees. Plants. 2020; 9(9):1104. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9091104
Chicago/Turabian StyleConesa, María R., Wenceslao Conejero, Juan Vera, and M. Carmen Ruiz-Sánchez. 2020. "Effects of Postharvest Water Deficits on the Physiological Behavior of Early-Maturing Nectarine Trees" Plants 9, no. 9: 1104. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9091104