Effect of Online Home-Based Training on Functional Capacity and Strength in Two CKD Patients: A Case Study
Abstract
:1. Introduction
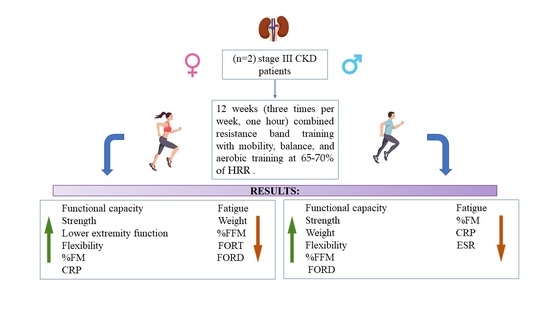
2. Materials and Methods
2.1. Intervention
2.2. Assessments
2.3. Functional and Psychological Evaluation
3. Results
3.1. Functional Evaluations
3.2. Anthropometric Assessment and Laboratory Parameters
3.3. Questionnaires Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inker, L.A.; Grams, M.E.; Levey, A.S.; Coresh, J.; Cirillo, M.; Collins, J.F.; Gansevoort, R.T.; Gutierrez, O.M.; Hamano, T.; Heine, G.H.; et al. Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium. Am. J. Kidney Dis. 2019, 73, 206–217. [Google Scholar] [CrossRef]
- Sabatino, A.; D’Alessandro, C.; Regolisti, G.; di Mario, F.; Guglielmi, G.; Bazzocchi, A.; Fiaccadori, E. Muscle mass assessment in renal disease: The role of imaging techniques. Quant. Imaging Med. Surg. 2020, 10, 1672–1686. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Enoki, Y.; Maruyama, T. Sarcopenia in Chronic Kidney Disease: Factors, Mechanisms, and Therapeutic Interventions. Biol. Pharm. Bull. 2019, 42, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Corsi Romanelli, M.M. Sarcopenia in Chronic Kidney Disease: Focus on Advanced Glycation End Products as Mediators and Markers of Oxidative Stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Rovella, V.; Cusumano, A.; Di Daniele, N.; Casasco, M. Beneficial effects of physical activity on uremic sarcopenia. Med. Dello Sport 2018, 71, 370–392. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef] [Green Version]
- Noce, A.; Romani, A.; Bernini, R. Dietary Intake and Chronic Disease Prevention. Nutrients 2021, 13, 1358. [Google Scholar] [CrossRef]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef]
- Nishi, H.; Takemura, K.; Higashihara, T.; Inagi, R. Uremic Sarcopenia: Clinical Evidence and Basic Experimental Approach. Nutrients 2020, 12, 1814. [Google Scholar] [CrossRef]
- Grazioli, E.; Romani, A.; Marrone, G.; Di Lauro, M.; Cerulli, C.; Urciuoli, S.; Murri, A.; Guerriero, C.; Tranchita, E.; Tesauro, M.; et al. Impact of Physical Activity and Natural Bioactive Compounds on Endothelial Dysfunction in Chronic Kidney Disease. Life 2021, 11, 841. [Google Scholar] [CrossRef]
- Johansen, K.L.; Painter, P. Exercise in individuals with CKD. Am. J. Kidney Dis. 2012, 59, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkman, D.L.; Ramick, M.G.; Muth, B.J.; Stock, J.M.; Pohlig, R.T.; Townsend, R.R.; Edwards, D.G. Effects of aerobic exercise on vascular function in nondialysis chronic kidney disease: A randomized controlled trial. Am. J. Physiol. Renal Physiol. 2019, 316, F898–F905. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; Williams, A.D.; Levinger, I.; Selig, S.; Howden, E.; Coombes, J.S.; Fassett, R.G. Exercise & Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J. Sci. Med. Sport 2013, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; Burris, D.D.; Lucas, F.L.; Crocker, G.A.; Wasserman, J.C. Effects of a renal rehabilitation exercise program in patients with CKD: A randomized, controlled trial. Clin. J. Am. Soc. Nephrol. 2014, 9, 2052–2058. [Google Scholar] [CrossRef] [Green Version]
- Senthil Kumar, T.G.; Soundararajan, P.; Maiya, A.G.; Ravi, A. Effects of graded exercise training on functional capacity, muscle strength, and fatigue after renal transplantation: A randomized controlled trial. Saudi J. Kidney Dis. Transpl. 2020, 31, 100–108. [Google Scholar] [CrossRef]
- Bossola, M.; Pellu, V.; Di Stasio, E.; Tazza, L.; Giungi, S.; Nebiolo, P.E. Self-reported physical activity in patients on chronic hemodialysis: Correlates and barriers. Blood Purif. 2014, 38, 24–29. [Google Scholar] [CrossRef]
- Goodman, E.D.; Ballou, M.B. Perceived barriers and motivators to exercise in hemodialysis patients. Nephrol. Nurs. J. 2004, 31, 23–29. [Google Scholar]
- Delgado, C.; Johansen, K.L. Barriers to exercise participation among dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- Kontos, P.C.; Miller, K.L.; Brooks, D.; Jassal, S.V.; Spanjevic, L.; Devins, G.M.; De Souza, M.J.; Heck, C.; Laprade, J.; Naglie, G. Factors influencing exercise participation by older adults requiring chronic hemodialysis: A qualitative study. Int. Urol. Nephrol. 2007, 39, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; You, L.M.; Lou, T.Q.; Chen, N.C.; Lai, D.Y.; Liang, Y.Y.; Li, Y.N.; Gu, Y.M.; Lv, S.F.; Zhai, C.Q. Development and psychometric evaluation of the Dialysis patient-perceived Exercise Benefits and Barriers Scale. Int. J. Nurs. Stud. 2010, 47, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Heiwe, S.; Jacobson, S.H. Exercise training in adults with CKD: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 64, 383–393. [Google Scholar] [CrossRef]
- Lasaponara, S.; Marson, F.; Doricchi, F.; Cavallo, M. A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far. Brain Sci. 2021, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, E.; Cerulli, C.; Dimauro, I.; Moretti, E.; Murri, A.; Parisi, A. New Strategy of Home-Based Exercise during Pandemic COVID-19 in Breast Cancer Patients: A Case Study. Sustainability 2020, 12, 6940. [Google Scholar] [CrossRef]
- Spanakis, M.; Roubedaki, M.; Tzanakis, I.; Zografakis-Sfakianakis, M.; Patelarou, E.; Patelarou, A. Impact of Adverse Drug Reactions in Patients with End Stage Renal Disease in Greece. Int. J. Environ. Res. Public Health 2020, 17, 9101. [Google Scholar] [CrossRef]
- Arroyo Monterroza, D.A.; Castro Bolivar, J.F. Pharmaceutical care practice in patients with chronic kidney disease. Farm. Hosp. 2017, 41, 137–149. [Google Scholar] [CrossRef]
- Gaman, M.A.; Epingeac, M.E.; Diaconu, C.C.; Gaman, A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes 2020, 11, 193–201. [Google Scholar] [CrossRef]
- Pavlatou, M.G.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef]
- Bucar Pajek, M.; Cuk, I.; Leskosek, B.; Mlinsek, G.; Buturovic Ponikvar, J.; Pajek, J. Six-Minute Walk Test in Renal Failure Patients: Representative Results, Performance Analysis and Perceived Dyspnea Predictors. PLoS ONE 2016, 11, e0150414. [Google Scholar] [CrossRef] [Green Version]
- Leal, V.O.; Mafra, D. Handgrip strength evaluation in CKD: Do we have enough evidence? J. Bras. Nephrol. 2020, 42, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Pols, M.A.; Peeters, P.H.; Bueno-De-Mesquita, H.B.; Ocke, M.C.; Wentink, C.A.; Kemper, H.C.; Collette, H.J. Validity and repeatability of a modified Baecke questionnaire on physical activity. Int. J. Epidemiol. 1995, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Alhaji, M.M.; Tan, J.; Hamid, M.R.; Timbuak, J.A.; Naing, L.; Tuah, N.A. Determinants of quality of life as measured with variants of SF-36 in patients with predialysis chronic kidney disease. Saudi Med. J. 2018, 39, 653–661. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Cheema, B.S.; Chan, D.; Fahey, P.; Atlantis, E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: A systematic review and meta-analysis. Sports Med. 2014, 44, 1125–1138. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, D.H.; Min, J.; Jeon, J.Y. Handgrip Strength as a Predictor of All-Cause Mortality in Patients with Chronic Kidney Disease Undergoing Dialysis: A Meta-Analysis of Prospective Cohort Studies. J. Ren. Nutr. 2019, 29, 471–479. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.S.; Jung, S.W.; Hwang, H.S.; Moon, J.Y.; Jeong, K.H.; Lee, S.H.; Lee, S.Y.; Ko, G.J.; Lee, D.Y.; et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020, 21, 166. [Google Scholar] [CrossRef]
- Amparo, F.C.; Cordeiro, A.C.; Carrero, J.J.; Cuppari, L.; Lindholm, B.; Amodeo, C.; Kamimura, M.A. Malnutrition-inflammation score is associated with handgrip strength in nondialysis-dependent chronic kidney disease patients. J. Ren. Nutr. 2013, 23, 283–287. [Google Scholar] [CrossRef]
- Zheng, W.; McLerran, D.F.; Rolland, B.; Zhang, X.; Inoue, M.; Matsuo, K.; He, J.; Gupta, P.C.; Ramadas, K.; Tsugane, S.; et al. Association between body-mass index and risk of death in more than 1 million Asians. N. Engl. J. Med. 2011, 364, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G.; D’Este, C. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, Y.Y.; Lee, K.B.; Rhee, E.J.; Park, C.Y.; Chang, Y.; Ryu, S. Chronic kidney disease and high eGFR according to body composition phenotype in adults with normal BMI. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1088–1095. [Google Scholar] [CrossRef]
- Lin, T.Y.; Peng, C.H.; Hung, S.C.; Tarng, D.C. Body composition is associated with clinical outcomes in patients with non-dialysis-dependent chronic kidney disease. Kidney Int. 2018, 93, 733–740. [Google Scholar] [CrossRef]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazioli, E.; Tranchita, E.; Marrone, G.; Urciuoli, S.; Di Lauro, M.; Cerulli, C.; Piacentini, N.; Murri, A.; Celotto, R.; Romani, A.; et al. The Impact of Functional Bars and Adapted Physical Activity on Quality of Life in Chronic Kidney Disease: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 3281. [Google Scholar] [CrossRef]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Ferraro, B.; Galli, F.; Frei, B.; Kingdon, E.; Canestrari, F.; Rice-Evans, C.; Buoncristiani, U.; Davenport, A.; Moore, K.P. Peroxynitrite-induced oxidation of plasma lipids is enhanced in stable hemodialysis patients. Kidney Int. 2003, 63, 2207–2213. [Google Scholar] [CrossRef] [Green Version]
- Krata, N.; Zagozdzon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Ther. Exp. 2018, 66, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [Green Version]
- Kao, T.W.; Lu, I.S.; Liao, K.C.; Lai, H.Y.; Loh, C.H.; Kuo, H.K. Associations between body mass index and serum levels of C-reactive protein. S. Afr. Med. J. 2009, 99, 326–330. [Google Scholar]
- Khera, A.; Vega, G.L.; Das, S.R.; Ayers, C.; McGuire, D.K.; Grundy, S.M.; de Lemos, J.A. Sex differences in the relationship between C-reactive protein and body fat. J. Clin. Endocrinol. Metab. 2009, 94, 3251–3258. [Google Scholar] [CrossRef] [Green Version]
- Afsar, B.; Siriopol, D.; Aslan, G.; Eren, O.C.; Dagel, T.; Kilic, U.; Kanbay, A.; Burlacu, A.; Covic, A.; Kanbay, M. The impact of exercise on physical function, cardiovascular outcomes and quality of life in chronic kidney disease patients: A systematic review. Int. Urol. Nephrol. 2018, 50, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.N.; Zhu, S.; Cooper, N.; Roderick, P.; Alwan, N.; Tarrant, C.; Ziauddeen, N.; Yao, G.L. Impact of Covid-19 on health-related quality of life of patients: A structured review. PLoS ONE 2021, 16, e0259164. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, A.; Buonsenso, A.; Baralla, F.; Grazioli, E.; Di Martino, G.; Lecce, E.; Calcagno, G.; Fiorilli, G. Psychological Impact of the Quarantine-Induced Stress during the Coronavirus (COVID-19) Outbreak among Italian Athletes. Int. J. Environ. Res. Public Health 2020, 17, 8867. [Google Scholar] [CrossRef]
- Castaneda-Babarro, A.; Arbillaga-Etxarri, A.; Gutierrez-Santamaria, B.; Coca, A. Physical Activity Change during COVID-19 Confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Lugo, A.; Amerio, A.; Borroni, E.; Bosetti, C.; Carreras, G.; Cavalieri d’Oro, L.; Colombo, P.; Fanucchi, T.; Ghislandi, S.; et al. COVID-19 lockdown impact on lifestyle habits of Italian adults. Acta Bio-Med. Atenei Parm. 2020, 91, 87–89. [Google Scholar] [CrossRef]
- Odden, M.C.; Chertow, G.M.; Fried, L.F.; Newman, A.B.; Connelly, S.; Angleman, S.; Harris, T.B.; Simonsick, E.M.; Shlipak, M.G.; Study, H. Cystatin C and measures of physical function in elderly adults: The Health, Aging, and Body Composition (HABC) Study. Am. J. Epidemiol. 2006, 164, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P. Interventions for Human Frailty: Physical Activity as a Model. Cold Spring Harb. Perspect. Med. 2016, 6, a025916. [Google Scholar] [CrossRef] [Green Version]
| Epidemiological Characteristics | Patient A | Patient B |
|---|---|---|
| Age (years) | 57 | 56 |
| Height (cm) | 163 | 184 |
| Weight (kg) | 71 | 83 |
| BMI (kg/m2) | 27.1 | 24.4 |
| Gender | F | M |
| Stage of CKD | IIIb | IIIb |
| Primary cause of CKD | nephroangiosclerosis | chronic pyelonephritis |
| Pharmacological treatment | Beta blockers (5 mg/day), angiotensin II type 1 receptor blockers (40 mg/day) | Alpha blockers (2 mg/day), beta blockers (5 mg/day), angiotensin-converting enzyme inhibitors (5 mg/day) |
| Functional Tests | Patient | T0 | T1 | % |
|---|---|---|---|---|
| 6MWT (m) | A | 500 | 515 | +3% |
| B | 750 | 790 | +5.3% | |
| 6MWT Borg | A | 4 | 2 | −50% |
| B | 5 | 4.5 | −10% | |
| HST-R (kg) | A | 26.1 | 29.6 | +13.4% |
| B | 51.8 | 61.7 | +19.1% | |
| HST-L (kg) | A | 18.9 | 27 | +42.8% |
| B | 47.7 | 53.9 | +12.9% | |
| SPPB (score) | A | 10 | 12 | +20% |
| B | 12 | 12 | 0% | |
| S&R (cm) | A | 3 | 3.5 | +16.6% |
| Anthropometric Assessment | Patient | T0 | T1 | % |
|---|---|---|---|---|
| Weight (kg) | A | 71.8 | 71.5 | −0.4% |
| B | 83.1 | 83.2 | +0.1% | |
| BMI (kg/m2) | A | 27.1 | 26.9 | −0.7% |
| B | 24.5 | 24.6 | +0.4% | |
| FM (%) | A | 34.9 | 35.5 | +1.7% |
| B | 24.1 | 18.4 | −23.6% | |
| FFM (%) | A | 46.9 | 46.2 | −1.4% |
| B | 63.1 | 67.9 | +7.6% | |
| TBW (%) | A | 47.6 | 47.3 | −0.6 |
| B | 55.5 | 61.5 | +10.8% | |
| ECW (%) | A | 48.0 | 46.1 | −4% |
| B | 49.8 | 43.4 | −13% | |
| ICW (%) | A | 52.0 | 53.9 | +3.5% |
| B | 50.2 | 56.6 | +12.7% | |
| BCM (%) | A | 53.4 | 51.3 | −2.9% |
| B | 56.2 | 49.3 | −12.2% | |
| BCMI | A | 9.4 | 8.9 | −5.3 |
| B | 10.5 | 9.9 | −5.7% | |
| SMMI (kg/h2) | A | 7.5 | 7.1 | −5.3% |
| B | 9.1 | 10.5 | +15.4% | |
| Waist circumferences (cm) | A | 92.9 | 93.1 | +0.2% |
| B | 106.1 | 105.5 | −0.5% |
| Laboratory Parameters | Patient | T0 | T1 | % |
|---|---|---|---|---|
| CRP (mg/L) | A | 5.1 | 5.6 | 9.8% |
| B | 1.4 | 0.8 | −42.8% | |
| ESR (mm/h) | A | 45 | 45 | 0% |
| B | 20 | 18 | −10% | |
| Albumin (g/dL) | A | 4.5 | 4.6 | + 2% |
| B | 4.5 | 4.2 | −6% | |
| Glycemia (mg/dL) | A | 89 | 99 | +9.7% |
| B | 75 | 78 | +4.2% | |
| Total cholesterol (g/dL) | A | 230 | 195 | −15% |
| B | 245 | 200 | −18% | |
| LDL-cholesterol (mg/dL) | A | 164 | 127 | −21.6% |
| B | 141 | 161 | +14% | |
| HDL-cholesterol (mg/dL) | A | 52 | 47 | −9% |
| B | 58 | 50 | −12.7% | |
| Triglycerides (mg/dL) | A | 147 | 131 | −11.4% |
| B | 92 | 108 | +17.3% | |
| FORT (U) | A | 421 | 160 | −61.9% |
| B | 160 | 160 | 0% | |
| FORD (mmol/L Trolox) | A | 1.74 | 1.13 | −35% |
| B | 1.01 | 1.09 | 7.9% |
| Questionnaires Scores | Patient | T0 | T1 | % |
|---|---|---|---|---|
| SF-36 PF | A | 70 | 60 | −14.2% |
| B | 100 | 96 | −5% | |
| SF-36 RLP | A | 20 | 0 | −100% |
| B | 100 | 100 | 0% | |
| SF-36 RLE | A | 66.7 | 33.3 | −50% |
| B | 100 | 100 | 0% | |
| SF-36 V (E/F) | A | 50 | 45 | −10% |
| B | 85 | 80 | −5.8% | |
| SF-36 EWB | A | 56 | 48 | −14.2% |
| B | 88 | 88 | 0% | |
| SF-36 SF | A | 50 | 50 | 0% |
| B | 87.5 | 100 | +14.2% | |
| SF-36 P | A | 45 | 45 | 0% |
| B | 80 | 90 | +12.5% | |
| SF-36 GH | A | 40 | 30 | −25% |
| B | 75 | 75 | 0% | |
| SF-36 HC | A | 50 | 50 | 0% |
| B | 50 | 75 | +50% | |
| BAECKE TOT | A | 7.6 | 7.8 | +2.6% |
| B | 8.1 | 8.6 | +6.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, G.; Grazioli, E.; Tranchita, E.; Parisi, A.; Cerulli, C.; Murri, A.; Minganti, C.; Di Lauro, M.; Piacentini, N.; Galiuto, L.; et al. Effect of Online Home-Based Training on Functional Capacity and Strength in Two CKD Patients: A Case Study. Healthcare 2022, 10, 572. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare10030572
Marrone G, Grazioli E, Tranchita E, Parisi A, Cerulli C, Murri A, Minganti C, Di Lauro M, Piacentini N, Galiuto L, et al. Effect of Online Home-Based Training on Functional Capacity and Strength in Two CKD Patients: A Case Study. Healthcare. 2022; 10(3):572. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare10030572
Chicago/Turabian StyleMarrone, Giulia, Elisa Grazioli, Eliana Tranchita, Attilio Parisi, Claudia Cerulli, Arianna Murri, Carlo Minganti, Manuela Di Lauro, Nicolò Piacentini, Leonarda Galiuto, and et al. 2022. "Effect of Online Home-Based Training on Functional Capacity and Strength in Two CKD Patients: A Case Study" Healthcare 10, no. 3: 572. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare10030572