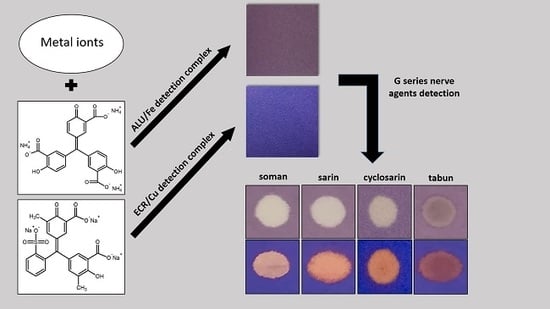
Detection Papers with Metal Complexes with Triphenylmethane Dyes for the Detection of G-Series Nerve Agents (Sarin, Soman, Cyclosarin) in the Liquid Phase
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Preparation of Detection Papers
- 1
- 0.05% of ALU, 0.02% of ferric chloride hexahydrate, and 10% of glycerol in distilled water,
- 2
- 0.15% of ECR, 0.5% of copper(II) chloride, and 10% of glycerol in distilled water.
2.3. Functionality Testing
3. Results and Discussion
3.1. Pilot Tests
3.2. pH Study of Color Complexes
3.3. Detection/Reaction Principle
3.4. Color Reactions with CWA, and Interferences
3.5. Color Stability
3.6. Limit of Detection
3.7. Stability of Detection Papers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costanzi, S.; Machado, J.H.; Mitchell, M. Nerve agents: What they are, how they work, how to counter them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Potential Military Chemical (Biological) Agents and Compounds; Field Manual FM 3-11.9; Exidyne: Wentzeville, MO, USA, 2005.
- Franke, S. Lehrbuch der Militärchemie; Militärverlag der DDR: Berlin, Germany, 1977; Volume 2v. [Google Scholar]
- Halámek, E.; Kobliha, Z.; Pitschmann, V. Analysis of Chemical Warfare Agents; University of Defence: Brno, Czech Republic, 2009. [Google Scholar]
- Thoraval, D.; Bovenkamp, J.W. Paper Chemical Agent Detectors, EP 0 334 668 B1, 23.12. 1992. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=2ahUKEwix0eLj6onmAhXhx6YKHdJhANcQFjACegQIBRAC&url=https%3A%2F%2Fpatentimages.storage.googleapis.com%2Fpdfs%2F57050eab456edfc3d6e9%2FEP0334668B1.pdf&usg=AOvVaw3RvGj0EAJta9K2rwuhUDtY (accessed on 28 November 2019).
- Galan-Freyle, N.J.; Figueroa-Navedo, A.M.; Pacheco-Londoño, Y.C.; Ortiz-Rivera, W.; Pacheco-Londoño, L.C.; Hernández-Rivera, S.P. Chemometrics-enhanced fiber optic Raman detection, discrimination and quantification of chemical agents simulants concealed in commercial bottles. Anal. Chem. Res. 2014, 2, 15–22. [Google Scholar] [CrossRef]
- Hu, G.; Xiong, W.; Luo, H.; Shi, H.; Li, Z.; Shen, J.; Fang, X.; Xu, B.; Zhang, J. Raman spectroscopic detection for simulants of chemical warfare agents using a spatial heterodyne spectrometer. Appl. Spectrosc. 2018, 72, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Royo, S.; Martinez-Máñez, R.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. Chromogenic and fluorogenic reagens for chemical warfare nerve agents detection. Chem. Commun. 2007, 4839–4847. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Yoon, J. Recent advances in the developments of chromophore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef]
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric sensor arrays for the detection and identification of chemical weapons and explosives. Crit. Rev. Anal. Chem. 2017, 47, 138–153. [Google Scholar] [CrossRef]
- Pitschmann, V.; Matějovský, L.; Lunerová, K.; Dymák, M.; Urban, M.; Králík, L. Detection papers with chromogenic chemosensors for direct visual detection and distinction of liquid chemical warfare agents. Chemosensors 2019, 7, 30. [Google Scholar] [CrossRef]
- Knapton, D.; Burnworth, M.; Rowan, S.J.; Weder, C. Fluorescent organometallic sensor for the detection of chemical warfare-agent mimics. Angew. Chem. Int. Ed. 2006, 45, 5825–5829. [Google Scholar] [CrossRef]
- Sarkar, S.; Mondal, A.; Tiwari, A.K.; Shunmugam, R. Unique emission from norbornene derived terpyridine—A selective chemodosimeter for G-type nerve agent surrogates. Chem. Commun. 2012, 48, 4223–4225. [Google Scholar] [CrossRef]
- Ordroneau, L.; Carella, A.; Pohanka, M.; Simonato, J.P. Chromogenic detection of sarin by discolouring decomplexation of a metal coordination complex. Chem. Commun. 2013, 49, 8946–8948. [Google Scholar] [CrossRef]
- Maza, W.A.; Vetromile, C.M.; Kim, C.; Xu, X.; Zhang, X.P.; Larsen, R.W. Spectroscopic investigation of the noncovalent association of nerve agent simulant diisopropyl methylphosphonate (DIMP) with zinc (II) porphyrins. J. Phys. Chem. A 2013, 117, 11308–11315. [Google Scholar] [CrossRef] [PubMed]
- Borba-Bon, A.; Costero, A.M.; Gil, S.; Sancenón, F.; Martinez-Máñez, R. Chromo-fluorogenic BODIPY complexes for selective of V-type nerve agent surrogates. Chem. Commun. 2014, 50, 13289–13291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Raviraju, G.; Rana, H.; Rao, V.K.; Gupta, A.K. Highly selective and sensitive chromogenic detection of nerve agents (sarin, tabun and VX): A multianalyte detection approach. Chem. Commun. 2017, 53, 12954–12957. [Google Scholar] [CrossRef] [PubMed]
- Sheet, K.S.; Sen, B.; Khatua, S. Organoiridium(III) complexes as luminiscence color swithing probes for selective detection of nerve agent simulant in solution and vapor phase. Inorg. Chem. 2019, 58, 3635–3645. [Google Scholar] [CrossRef] [PubMed]
- Ishida, R.; Narita, H.; Tonosaki, K. Copper(II)-2,2´-bipyridine-eriochrome cyanine R ternary complex and its application to the spectrophotometric determination of copper(II). Bull. Chem. Soc. Jpn. 1978, 51, 1751–1754. [Google Scholar] [CrossRef]
- Dacres, H.; Narayanaswamy, R. Evaluation of cooper(II) eriochrome cyanine R (ECR) complex immobilized in anion exchange membrane as a potential nitric oxide optical sensor. Aust. J. Chem. 2008, 61, 189–196. [Google Scholar] [CrossRef]
- Dapson, R.; Horobin, R.W.; Kierman, J. Hematoxylin shortages: Their causes and duration, and other dyes that can replace hemalum in routine hematoxylin and eosin staining. Biotech. Histochem. 2010, 85, 55–63. [Google Scholar] [CrossRef]
- Sarsam, L.A.; Bashir, W.A. Spectrophotometric determination of scandium(III) with eriochrome cyanine R and cetylpyridinium chloride—Application to waters and synthetic alloys. J. Raf. Sci. 2009, 20, 48–65. [Google Scholar]
- Langmyhr, F.J.; Stumpe, T. Complex formation of iron(III) with eriochrome cyanine R. Anal. Chim. Acta 1965, 32, 535–543. [Google Scholar] [CrossRef]
- Majeed, A.; Asma, D.R.N.; Sana-Ullah, M. Spectrophotometric determination of iron with xylenol orange. J. Chem. Soc. Pak. 1996, 18, 197–200. [Google Scholar]
- Mizuguchi, H.; Yotsuyanagi, T. Visual threshold detection of trace metal ions using a bi-functional metallochromic reagent. Anal. Sci. 2001, 17, i1687–i1689, Supplement. [Google Scholar]
- Barghouthi, Z.; Amereih, S. Spectrophotometric determination of fluoride in drinking water using aluminium complexes of triphenylmethane dyes. Water SA 2012, 38, 543–548. [Google Scholar] [CrossRef]








| Complex | Original Colour | Change of Colour with CWA in Liquid Phase | ||||
|---|---|---|---|---|---|---|
| GA | GB | VX | HD | L | ||
| ALU/Al3+ | Red | - | lightening | - | - | - |
| ALU/Fe3+ | violet | - | lightening | - | - | orange |
| ALU/Zn2+ | Red | - | lightening | - | - | - |
| ECR/Cu2+ | Blue | orange | orange | - | - | orange |
| ECR/Al3+ | violet | orange | orange | - | - | orange |
| XO/Fe3+ | Blue | - | pink | - | - | orange |
| XO/Al3+ | Pink | - | yellow | - | - | - |
| XO/Zn2+ | Red | yellow | yellow | Pink | pink | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobotka, M.; Pitschmann, V.; Matějovský, L. Detection Papers with Metal Complexes with Triphenylmethane Dyes for the Detection of G-Series Nerve Agents (Sarin, Soman, Cyclosarin) in the Liquid Phase. Chemosensors 2019, 7, 59. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors7040059
Lobotka M, Pitschmann V, Matějovský L. Detection Papers with Metal Complexes with Triphenylmethane Dyes for the Detection of G-Series Nerve Agents (Sarin, Soman, Cyclosarin) in the Liquid Phase. Chemosensors. 2019; 7(4):59. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors7040059
Chicago/Turabian StyleLobotka, Martin, Vladimír Pitschmann, and Lukáš Matějovský. 2019. "Detection Papers with Metal Complexes with Triphenylmethane Dyes for the Detection of G-Series Nerve Agents (Sarin, Soman, Cyclosarin) in the Liquid Phase" Chemosensors 7, no. 4: 59. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors7040059