Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde as a Novel Nanocomposite for the Efficient Removal of Cd(II) and Cu(II) Ions from Aqueous Media
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis
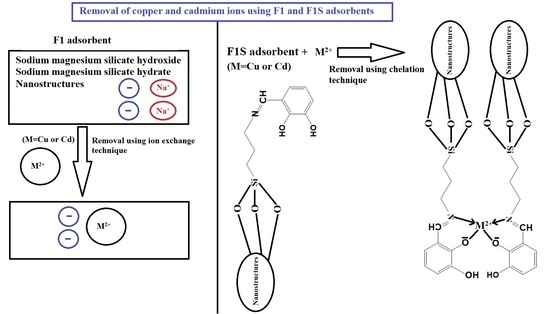
2.2.1. Synthesis of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures
2.2.2. Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde
2.3. Instrumentation
2.4. Removal of Copper and Cadmium Ions from Aqueous Media
3. Results and Discussion
3.1. Characterization of the Synthesized Samples
3.2. Removal of Cd(II) and Cu(II) Ions
3.2.1. Effect of pH
3.2.2. Effect of Time
3.2.3. Effect of Temperature
3.2.4. Effect of Concentration
3.2.5. Effect of Desorption and Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, H.; Min, X.; Tang, C.J.; Sillanpää, M.; Zhao, F. Recent Advances in Membrane Filtration for Heavy Metal Removal from Wastewater: A Mini Review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Sinharoy, A.; Kumar, M.; Chaudhuri, R.; Saikia, S.; Pakshirajan, K. Simultaneous Removal of Selenite and Heavy Metals from Wastewater and Their Recovery as Nanoparticles Using an Inverse Fluidized Bed Bioreactor. J. Clean. Prod. 2022, 376, 134248. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Y.; Sun, W.; Xie, J.; Wang, M.; Guo, Z. A Novel Electrocoagulation Process with Centrifugal Electrodes for Wastewater Treatment: Electrochemical Behavior of Anode and Kinetics of Heavy Metal Removal. Chemosphere 2023, 310, 136862. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Wang, H.; Xia, C.; Yan, P.; Zhang, Q.; Meng, Z. Adsorption Performance of Layered Double Hydroxides for Heavy Metals Removal in Soil with the Presence of Microplastics. J. Environ. Chem. Eng. 2022, 10, 108733. [Google Scholar] [CrossRef]
- Ghamari, F.; Negar, Z.A.; Arjomandi, J.; Shi, H. Novel Polyaniline/8-Hydroxyquinoline Composite Electrode Materials for Simultaneous Electrochemical Removal of Heavy Metal Ions from Water Resources. J. Environ. Chem. Eng. 2022, 10, 108830. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, M.; Fan, X.; Meng, Z.; Zhang, Q.; Lv, F. MoS42-Intercalated Magnetic Layered Double Hydroxides for Effective Removal and Expedient Recovery of Heavy Metals from Soil. Chem. Eng. J. 2023, 454, 139965. [Google Scholar] [CrossRef]
- Mallik, A.K.; Kabir, S.F.; Bin Abdur Rahman, F.; Sakib, M.N.; Efty, S.S.; Rahman, M.M. Cu(II) Removal from Wastewater Using Chitosan-Based Adsorbents: A Review. J. Environ. Chem. Eng. 2022, 10, 108048. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/Desorption of Cd(II), Cu(II) and Pb(II) Using Chemically Modified Orange Peel: Equilibrium and Kinetic Studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Chen, X.; Tong, A. Modification of Silica Nanoparticles with Fluorescein Hydrozide for Cu(II) Sensing. Dyes Pigment. 2012, 95, 776–783. [Google Scholar] [CrossRef]
- Taseidifar, M.; Makavipour, F.; Pashley, R.M.; Rahman, A.F.M.M. Removal of Heavy Metal Ions from Water Using Ion Flotation. Environ. Technol. Innov. 2017, 8, 182–190. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Shao, S.; Lai, C.; Wu, D.; Xu, J.; Luo, X.; Xu, D.; Liang, H.; Zhu, X. Highly Permeable Positively Charged Nanofiltration Membranes with Multilayer Structures for Multiple Heavy Metal Removals. Desalination 2023, 548, 116266. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Naglah, A.M.; Saad, F.A.; Abdelrahman, E.A. Modification of Sodium Aluminum Silicate Hydrate by Thioglycolic Acid as a New Composite Capable of Removing and Preconcentrating Pb(II), Cu(II), and Zn(II) Ions from Food and Water Samples. Arab. J. Chem. 2022, 15, 104178. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; AlSalem, H.S.; Alshalawi, A.F.; Naglah, A.M.; Al-Anwar, A.; Abdelrahman, E.A. Facile Synthesis of a Novel Nanocomposite for Determination of Mercury and Copper Ions in Food and Water Samples. Arab. J. Chem. 2022, 15, 104113. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Naglah, A.M.; Saad, F.A.; Abdelrahman, E.A. Modification of Silica Nanoparticles with 1-Hydroxy-2-Acetonaphthone as a Novel Composite for the Efficient Removal of Ni(II), Cu(II), Zn(II), and Hg(II) Ions from Aqueous Media. Arab. J. Chem. 2022, 15, 104010. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Abou El-Reash, Y.G.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of Rice Husk and Waste Aluminum Cans for the Synthesis of Some Nanosized Zeolite, Zeolite/Zeolite, and Geopolymer/Zeolite Products for the Efficient Removal of Co(II), Cu(II), and Zn(II) Ions from Aqueous Media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, E.A.; Alharbi, A.; Subaihi, A.; Hameed, A.M.; Almutairi, M.A.; Algethami, F.K.; Youssef, H.M. Facile Fabrication of Novel Analcime/Sodium Aluminum Silicate Hydrate and Zeolite Y/Faujasite Mesoporous Nanocomposites for Efficient Removal of Cu(II) and Pb(II) Ions from Aqueous Media. J. Mater. Res. Technol. 2020, 9, 7900–7914. [Google Scholar] [CrossRef]
- Gendy, E.A.; Ifthikar, J.; Ali, J.; Oyekunle, D.T.; Elkhlifia, Z.; Shahib, I.I.; Khodair, A.I.; Chen, Z. Removal of Heavy Metals by Covalent Organic Frameworks (COFs): A Review on Its Mechanism and Adsorption Properties. J. Environ. Chem. Eng. 2021, 9, 105687. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of Zeolites by In-Situ Conversion of Geopolymers and Their Performance of Heavy Metal Ion Removal in Wastewater: A Review. J. Clean. Prod. 2022, 349, 131441. [Google Scholar] [CrossRef]
- Azad, H.; Mohsennia, M.; Cheng, C.; Amini, A. Facile Fabrication of PVB-PVA Blend Polymer Nanocomposite for Simultaneous Removal of Heavy Metal Ions from Aqueous Solutions: Kinetic, Equilibrium, Reusability and Adsorption Mechanism. J. Environ. Chem. Eng. 2021, 9, 106214. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Efficient Removal of Heavy Metals from Artificial Wastewater Using Biochar. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100602. [Google Scholar] [CrossRef]
- Basu, H.; Saha, S.; Pimple, M.V.; Singhal, R.K. Novel Hybrid Material Humic Acid Impregnated Magnetic Chitosan Nano Particles for Decontamination of Uranium from Aquatic Environment. J. Environ. Chem. Eng. 2019, 7, 103110. [Google Scholar] [CrossRef]
- Haripriyan, U.; Gopinath, K.P.; Arun, J. Chitosan Based Nano Adsorbents and Its Types for Heavy Metal Removal: A Mini Review. Mater. Lett. 2022, 312, 131670. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Abdelrahman, E.A.; Hassanien, M.M.; Ibrahim, W.A. Application of Mesoporous Silica Nanoparticles Modified with Dibenzoylmethane as a Novel Composite for Efficient Removal of Cd(II), Hg(II), and Cu(II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2182–2196. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.; Cui, X.; Che, X.; Zhang, Q.; Zhong, J.; Jin, R.; Wang, L.; Luo, Y. Kinetics, Thermodynamics, and Equilibrium of As(III), Cd(II), Cu(II) and Pb(II) Adsorption Using Porous Chitosan Bead-Supported MnFe2O4 Nanoparticles. Int. J. Min. Sci. Technol. 2021, 31, 1107–1115. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. Magnetic Dithiocarbamate Functionalized Reduced Graphene Oxide for the Removal of Cu(II), Cd(II), Pb(II), and Hg(II) Ions from Aqueous Solution: Synthesis, Adsorption, and Regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous Removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) Ions from Aqueous Solutions via Adsorption on FAU-Type Zeolites Prepared from Coal Fly Ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Hang, Y.; Si, Y.; Zhou, Q.; Yin, H.; Wang, A.; Cao, A. Morphology-Controlled Synthesis of Calcium Titanate Particles and Adsorption Kinetics, Isotherms, and Thermodynamics of Cd(II), Pb(II), and Cu(II) Cations. J. Hazard. Mater. 2019, 380, 120789. [Google Scholar] [CrossRef]
- Ramana, D.K.V.; Yu, J.S.; Seshaiah, K. Silver Nanoparticles Deposited Multiwalled Carbon Nanotubes for Removal of Cu(II) and Cd(II) from Water: Surface, Kinetic, Equilibrium, and Thermal Adsorption Properties. Chem. Eng. J. 2013, 223, 806–815. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from Aqueous Single Metal Solutions by Guanyl-Modified Cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef]




















| Parameter | Co (mg/L) | V (mL) | M (g) | Vd (mL) |
|---|---|---|---|---|
| pH (2.5–7.5) | 200 | 40 | 0.04 | --- |
| Time (10–120 min) | 200 | 40 | 0.04 | --- |
| Concentration (80–280 mg/L) | --- | 40 | 0.04 | --- |
| Desorption | 5 | 40 | 0.04 | 4 |
| Reusability | 5 | 40 | 0.04 | 4 |
| Surface Properties | Sample | |
|---|---|---|
| F1 | F1S | |
| BET surface area (m2/g) | 199.89 | 12.95 |
| Total pore volume (cc/g) | 0.2126 | 0.0172 |
| Average pore size (nm) | 2.1275 | 2.214 |
| Conditions | Qe (mg/g) | Constants | R2 | |||
|---|---|---|---|---|---|---|
| Pseudo-First-Order | Pseudo-Second-Order | k1 (1/min) | k2 (g/mg·min) | Pseudo-First-Order | Pseudo-Second-Order | |
| F1 + Cd(II) ions | 69.86 | 81.04 | 0.0161 | 0.00037 | 0.9809 | 0.9948 |
| F1S + Cd(II) ions | 126.52 | 157.23 | 0.0185 | 0.00021 | 0.9914 | 0.9946 |
| F1 + Cu(II) ions | 92.66 | 112.74 | 0.0154 | 0.00018 | 0.9890 | 0.9996 |
| F1S + Cu(II) ions | 113.02 | 179.86 | 0.0272 | 0.00043 | 0.9771 | 0.9967 |
| Conditions | ΔH° (kJ/mol) | ΔS° (kJ/mol Kelvin) | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| F1 + Cd(II) ions | −45.47 | 0.1555 | −91.79 | −93.35 | −94.91 | −96.46 |
| F1S + Cd(II) ions | −44.32 | 0.1399 | −86.01 | −87.40 | −88.80 | −90.20 |
| F1 + Cu(II) ions | −43.00 | 0.1444 | −86.03 | −87.47 | −88.91 | −90.36 |
| F1S + Cu(II) ions | −42.39 | 0.1267 | −80.16 | −81.42 | −82.69 | −83.96 |
| Conditions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | Qm (mg/g) | KF (mg/g)(L/mg)1/n | R2 | |
| F1 + Cd(II) ions | 89.44 | 0.0969 | 0.9948 | 84.42 | 51.98 | 0.8270 |
| F1S + Cd(II) ions | 155.04 | 0.2605 | 0.9929 | 161.37 | 86.21 | 0.9396 |
| F1 + Cu(II) ions | 103.73 | 0.3562 | 0.9989 | 101.95 | 82.79 | 0.9598 |
| F1S + Cu(II) ions | 177.94 | 1.124 | 0.9998 | 188.15 | 125.49 | 0.9180 |
| Adsorbent | Adsorption Capacity (mg/g) | Ref. | |
|---|---|---|---|
| Cd(II) | Cu(II) | ||
| Chitosan/MnFe2O4 composite | 9.73 | 43.94 | [25] |
| Dithiocarbamate/Fe3O4/reduced graphene oxide composite | 116.30 | 113.60 | [26] |
| FAU zeolite | 74.07 | 57.80 | [27] |
| Calcium titanate | 82.60 | 66.40 | [28] |
| Silver/multiwalled carbon nanotubes | 54.92 | 58.02 | [29] |
| Guanyl-modified cellulose | 68 | 83 | [30] |
| F1 | 89.44 | 103.73 | This study |
| F1S | 155.04 | 177.94 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wasidi, A.S.; Basha, M.T.; Alghanmi, R.M.; Al-Farraj, E.S.; Abdelrahman, E.A. Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde as a Novel Nanocomposite for the Efficient Removal of Cd(II) and Cu(II) Ions from Aqueous Media. Separations 2023, 10, 88. https://0-doi-org.brum.beds.ac.uk/10.3390/separations10020088
Al-Wasidi AS, Basha MT, Alghanmi RM, Al-Farraj ES, Abdelrahman EA. Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde as a Novel Nanocomposite for the Efficient Removal of Cd(II) and Cu(II) Ions from Aqueous Media. Separations. 2023; 10(2):88. https://0-doi-org.brum.beds.ac.uk/10.3390/separations10020088
Chicago/Turabian StyleAl-Wasidi, Asma S., Maram T. Basha, Reem M. Alghanmi, Eida S. Al-Farraj, and Ehab A. Abdelrahman. 2023. "Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde as a Novel Nanocomposite for the Efficient Removal of Cd(II) and Cu(II) Ions from Aqueous Media" Separations 10, no. 2: 88. https://0-doi-org.brum.beds.ac.uk/10.3390/separations10020088