Bio-Guided Fractionation of Oil Palm (Elaeis guineensis) Fruit and Interactions of Compounds with First-Line Antituberculosis Drugs against Mycobacterium tuberculosis H37Ra
Abstract
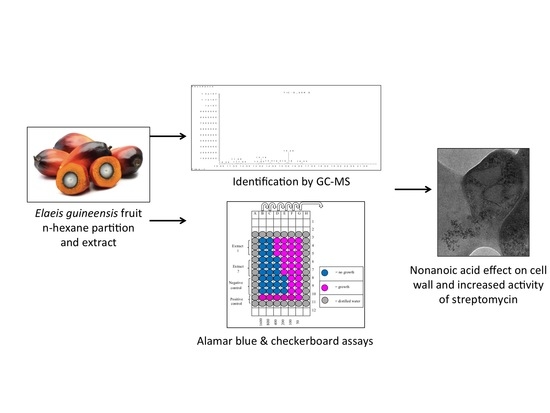
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Plant Extraction
2.3. Antimycobacterial Screening
2.3.1. MIC Assay
2.3.2. MBC Assay
2.4. Identification of Active Compound
2.4.1. Thin Layer Chromatography (TLC)
2.4.2. Column Chromatography
2.4.3. Gas Chromatography-Mass Spectrometry (GC-MS)
2.5. Drug Interaction
2.5.1. Checkerboard Assay
2.5.2. Time–Kill Assay
2.6. Transmission Electron Microscopy
2.7. Cytotoxicity Evaluation
3. Results and Discussion
3.1. Yield and Anti-Mycobacterial Activity of OPF Fractions and Partitions
3.2. MIC and MBC Screening of GC-MS Identified Compounds in n-hexane Fractions
3.3. Additive Interaction and Bactericidal Effect of Nonanoic Acid and Dodecanoic Acid with First-Line TB Drugs
3.4. Nonanoic Acid Has Relatively Low Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sulis, G.; Centis, R.; Sotgiu, G.; D’Ambrosio, L.; Pontali, E.; Spanevello, A.; Matteelli, A.; Zumla, A.; Migliori, G.B. Recent developments in the diagnosis and management of tuberculosis. NPJ Prim. Care. Respir. Med. 2016, 26, 16078. [Google Scholar] [CrossRef]
- Unissa, A.N.; Subbian, S.; Hanna, L.E.; Selvakumar, N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect. Genet. Evol. 2016, 45, 474–492. [Google Scholar] [CrossRef]
- Bollenbach, T. Antimicrobial interactions: Mechanisms and implications for drug discovery and resistance evolution. Curr. Opin. Microbiol. 2015, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-K.; Yusoff, K.; Mai, C.-W.; Lim, W.-M.; Yap, W.-S.; Lim, S.-H.; Lai, K.S. Additivity vs Synergism: Investigation of the Additive Interaction of Cinnamon Bark Oil and Meropenem in Combinatory Therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef] [Green Version]
- Kondo, E.; Kanai, K. The relationship between the chemical structure of fatty acids and their mycobactericidal activity. Jpn. J. Med. Sci. Biol. 1977, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, R.M. The Bactericidal Action of Low Molecular Weight Compounds on Mycobacterium tuberculosis. J. Appl. Bacteriol. 1964, 27. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Yew, V.W.C.; Awang, J.B.; Manaf, A.A.; Zaimah, R.; Nambiappan, B. The sustainability of oil palm industry in Malaysia: A comprehensive review. Int. J. Econ. Perspect. 2016, 10, 305–310. [Google Scholar]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Ramanathan, N.; Tan, E.; Loh, L.J.; Soh, B.S.; Yap, W.N. Tocotrienol is a cardioprotective agent against ageing-associated cardiovascular disease and its associated morbidities. Nutr. Metab. 2018, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Syarifah-Noratiqah, S.-B.; Zulfarina, M.S.; Ahmad, S.U.; Fairus, S.; Naina-Mohamed, I. The Pharmacological Potential of Oil Palm Phenolics (OPP) Individual Components. Int. J. Med. Sci. 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Chong, K.; Zakaria, Z.; Sasidharan, S.; Kalnisha, P.; Lachimanan, Y.; Ramanathan, S. Antimicrobial Activity of Elaeis guineensis Leaf. Pharmacologyonline 2007, 3, 379–386. [Google Scholar]
- Ohta, S.; Shiomi, Y.; Kawashima, A.; Aozasa, O.; Nakao, T.; Nagate, T.; Kitamura, K.; Miyata, H. Antibiotic effect of linolenic acid from Chlorococcum strain HS-101 and Dunaliella primolecta on methicillin-resistant Staphylococcus aureus. J. Appl. Phycol. 1995, 7, 121–127. [Google Scholar] [CrossRef]
- Sopirala, M.M.; Mangino, J.E.; Gebreyes, W.A.; Biller, B.; Bannerman, T.; Balada-Llasat, J.-M.; Pancholi, P. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother 2010, 54, 4678–4683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies-Methods and Approaches to Study the Interaction between Natural Products. Planta. Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [Green Version]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Tchinda, A.T.; Kapche, D.G.W.F.; Ngadjui, B.T.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, antimicrobial and antioxidant activity of eight compounds isolated from Entada abyssinica (Fabaceae). BMC Res. Notes. 2017, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, S.; Ibrahim, P.; Sadikun, A. Susceptibility of Mycobacterium tuberculosis to isoniazid and its derivative, 1-isonicotinyl-2-nonanoyl hydrazine: Investigation at cellular level. Tuberculosis 2004, 84. [Google Scholar] [CrossRef] [PubMed]
- Basyuni, M.; Amri, N.; Agustina, L.; Syahputra, I.; Arifiyanto, D. Characteristics of Fresh Fruit Bunch Yield and the Physicochemical Qualities of Palm Oil during Storage in North Sumatra, Indonesia. Indones. J. Chem. 2017, 17, 182. [Google Scholar] [CrossRef] [Green Version]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Pires, D.; Aínsa, J.A.; Gracia, B.; Mulhovo, S.; Duarte, A.; Anes, E.; Ferreira, M.U. Antimycobacterial evaluation and preliminary phytochemical investigation of selected medicinal plants traditionally used in Mozambique. J. Ethnopharmacol. 2011, 137, 114–120. [Google Scholar] [CrossRef]
- Esquivel-Ferriño, P.C.; Clemente-Soto, A.F.; Ramírez-Cabriales, M.Y.; Garza-González, E.; Álvarez, L.; Camacho-Corona, M.R. Volatile Constituents Identified in Hexane Extract of Citrus sinensis Peel and Anti-Mycobacterial Tuberculosis Activity of Some of its Constituents. Rev. Soc. Química. Mex. 2014, 58, 431–434. [Google Scholar]
- Sahin, N.; Kula, İ.; Erdogan, Y. Investigation of antimicrobial activities of nonanoic acid derivatives. Fresenius. Environ. Bull. 2006, 15, 141–143. [Google Scholar]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial Activity of Saturated Fatty Acids and Fatty Amines against Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, P.J.; Nikaido, H. The Envelope of Mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef]
- Chiaradia, L.; Lefebvre, C.; Parra, J.; Marcoux, J.; Burlet-Schiltz, O.; Etienne, G.; Tropis, M.; Daffé, M. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 2017, 7, 12807. [Google Scholar] [CrossRef] [Green Version]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018, 73, 1138–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morbidoni, H.R.; Vilchèze, C.; Kremer, L.; Bittman, R.; Sacchettini, J.C.; Jacobs, W.R. Dual Inhibition of Mycobacterial Fatty Acid Biosynthesis and Degradation by 2-Alkynoic Acids. Chem. Biol. 2006, 13, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, H.; Tomioka, H.; Yoneyama, T. Growth of group IV mycobacteria on medium containing various saturated and unsaturated fatty acids. Antimicrob. Agents Chemother. 1984, 26, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Ge, F.; Zeng, F.; Liu, S.; Guo, N.; Ye, H.; Song, Y.; Fan, J.; Wu, X.; Wang, X.; Deng, X.; et al. In vitro synergistic interactions of oleanolic acid in combination with isoniazid, rifampicin or ethambutol against Mycobacterium tuberculosis. J. Med. Microbiol. 2010, 59, 567–572. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, W., Jr.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Hill, R.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Pelargonic Acid (Nonanoic Acid) and Nonanoate Esters. Int. J. Toxicol. 2011, 30, 228S–269S. [Google Scholar] [CrossRef] [PubMed]


| OPF Partition/Drug | Crude Extract (g) | Partition Yield (g %) | MIC (µg/mL) |
|---|---|---|---|
| Methanol | 1709.73 | 228.28 (13) | 1600 |
| n-Hexane | 5.00 | 3.62 (73.3) | 800 |
| Chloroform | 1.38 | 0.11 (7.69) | 400 |
| Ethyl acetate | 1.27 | 0.03 (2.36) | 1600 |
| Butanol | 1.24 | 0.32 (25.74) | 1600 |
| Aqueous | 0.92 | 0.86 (93.17) | 1600 |
| Isoniazid | NA | NA | 0.78 |
| Retention time (min) | Peak Area | %of Total Peak Area | Compound Name | Library Matching (%) | Mol. Formula | Mol. Weight (g/mol) | CAS. No. | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 10.44 | 2171329 | 0.01% | Nonanoic acid | 93 | C9H18O2 | 158.24 | 112-05-0 | 50 | >400 |
| 10.72 | 1732053 | 0.01% | Decanoic acid | 87 | C10H20O2 | 172.26 | 334-48-5 | 50 | 400 |
| 11.43 | 3937048 | 0.01% | Dodecanoic acid | 98 | C12H24O2 | 200.32 | 143-07-7 | 50 | 200 |
| 12.25 | 2167712 | 0.01% | Tetradecanoic acid | 91 | C14H28O2 | 228.37 | 544-63-8 | 200 | ND |
| 13.05 | 12593009 | 0.08% | Hexadecanoic acid | 99 | C16H32O2 | 256.43 | 57-10-3 | 400 | ND |
| 13.89 | 16366821 | 0.04% | Oleic acid | 93 | C18H34O2 | 282.46 | 112-80-1 | 100 | ND |
| 14.53 | 10607471 | 0.03% | 9-Octadecenamide | 97 | C18H35NO | 281.48 | 301-02-0 | NI | ND |
| 16.33 | 4811228 | 0.01% | Squalene | 95 | C30H50 | 410.72 | 111-02-4 | NI | ND |
| 21.01 | 3391327 | 0.01% | Tri (2-ethyhexyl) trimelitate | 87 | C39H54O2 | 546.78 | 3319-31-1 | NI | ND |
| Combination | Individual MIC (µg/mL) | Combination MIC (µg/mL) | Fold Increase (+) or Decrease (−) in MIC | Individual FIC | FIC Index | Interaction | |
|---|---|---|---|---|---|---|---|
| Compound | Drug | Compound/Drug | Compound/Drug | Compound/Drug | Compound/Drug | ||
| Nonanoic acid | STR | 50/3.12 | 6.25/1.56 | +8/+2 | 0.125/0.5 | 0.625 | Additive |
| INH | 50/0.156 | 50/0.0098 | 1/+16 | 1/0.062 | 1.062 | Transition | |
| EMB | 50/3.125 | 100/0.391 | −2/+8 | 2/0.125 | 2.125 | Antagonistic | |
| RIF | 50/0.125 | 25/0.0625 | +2/+2 | 0.5/0.5 | 1 | Indifferent | |
| Decanoic acid | STR | 50/1.56 | 25/0.78 | +2/+2 | 0.5/0.5 | 1 | Indifferent |
| INH | 50/0.156 | 50/0.0097 | 1/+16 | 1/0.063 | 1.062 | Transition | |
| EMB | 50/3.125 | 50/0.39 | 1/+8 | 1/0.125 | 1.125 | Transition | |
| RIF | 50/0.125 | 25/0.031 | +2/+4 | 0.5/0.25 | 0.75 | Additive | |
| Dodecanoic acid | STR | 50/1.56 | 50/0.195 | 1/+8 | 1/0.125 | 1.125 | Transition |
| INH | 50/0.156 | 6.25/0.156 | +8/1 | 0.125/1 | 1.125 | Transition | |
| EMB | 50/3.125 | 6.25/3.125 | +8/1 | 0.125/1 | 1.125 | Transition | |
| RIF | 50/0.0625 | 6.25/0.0625 | +8/1 | 0.125/1 | 1.125 | Transition | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, Z.Y.; Sandanamsamy, S.; Ismail, N.N.; Mohamad, S.; Mohd Hanafiah, K. Bio-Guided Fractionation of Oil Palm (Elaeis guineensis) Fruit and Interactions of Compounds with First-Line Antituberculosis Drugs against Mycobacterium tuberculosis H37Ra. Separations 2021, 8, 19. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8020019
Chong ZY, Sandanamsamy S, Ismail NN, Mohamad S, Mohd Hanafiah K. Bio-Guided Fractionation of Oil Palm (Elaeis guineensis) Fruit and Interactions of Compounds with First-Line Antituberculosis Drugs against Mycobacterium tuberculosis H37Ra. Separations. 2021; 8(2):19. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8020019
Chicago/Turabian StyleChong, Zhen Yee, Sylvia Sandanamsamy, Nur Najihah Ismail, Suriyati Mohamad, and Khayriyyah Mohd Hanafiah. 2021. "Bio-Guided Fractionation of Oil Palm (Elaeis guineensis) Fruit and Interactions of Compounds with First-Line Antituberculosis Drugs against Mycobacterium tuberculosis H37Ra" Separations 8, no. 2: 19. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8020019