Spin Canting and Weak Ferromagnetism in a New 2D Coordination Polymer with the Co(II) Chain Bridged by a Single End-to-End Azide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization of Compound 1
2.2. Description of Structure of {[Co(N3)(bpmb)(H2O)2](NO3)·H2O}n (1)
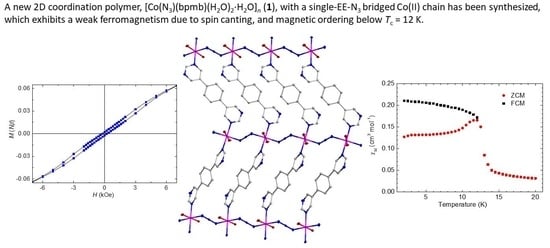
2.3. Magnetic Properties
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of {[Co(N3)(bpmb)(H2O)2](NO3)·H2O}n (1)
3.3. X-ray Crystallography
3.4. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal-organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.; Claridge, J.B.; Cussen, E.J.; Prior, T.J.; Rosseinsky, M.J. Design, Chirality, and Flexibility in Nanoporous Molecule-Based Materials. Acc. Chem. Res. 2005, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Wu, X.; Zhang, J.; Fu, R.; Hu, S.; Zhang, X. A 3D Canted Antiferromagnetic Porous Metal−Organic Framework with Anatase Topology through Assembly of an Analogue of Polyoxometalate. J. Am. Chem. Soc. 2005, 127, 16352–16353. [Google Scholar] [CrossRef]
- Palii, A.V.; Reu, O.S.; Ostrovsky, S.M.; Klokishner, S.I.; Tsukerblat, B.S.; Sun, Z.-M.; Mao, J.-G.; Prosvirin, A.V.; Zhao, H.-H.; Dunbar, K.R. A highly anisotropic cobalt (II)-based single-chain magnet: Exploration of spin canting in an antiferromagnetic array. J. Am. Chem. Soc. 2008, 130, 14729–14738. [Google Scholar] [CrossRef]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef]
- Guijarro, A.; Castillo, O.; Welte, L.; Calzolari, A.; Miguel, P.J.S.; Gómez-García, C.J.; Olea, D.; di Felice, R.; Gómez-Herrero, J.; Zamora, F. Conductive Nanostructures of MMX Chains. Adv. Funct. Mater. 2010, 20, 1451–1457. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Colacio, E.; Lloret, F.; Navarrete, M.; Romerosa, A.; Stoeckli-Evans, H.; Suarez-Varela, J. 2D and 3D coordination polymers based on 2,2′-bipyrimidine and cyanide bridging ligands incorporating coordinated and guest ammonia molecules. Synthesis, crystal structures, magnetic properties and thermal analysis of {[Ni(CN)4]2[(Ni(NH3)2)2(bpym)]·2H2O}n and {[Cu2(CN)2(bpym)]·NH3}n. New J. Chem. 2005, 29, 1189. [Google Scholar]
- De Munno, G.; Viterbo, D.; Caneschi, A.; Lloret, F.; Julve, M. A Giant Antiferromagnetic Interaction through the Bihydroxide Bridge (H3O2−). Inorg. Chem. 1994, 33, 1585–1586. [Google Scholar] [CrossRef]
- Okawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-Bridged Bimetallic Complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII, CoII; NH(prol)3+ = Tri(3-hydroxypropyl)ammonium) Exhibiting Coexistent Ferromagnetism and Proton Conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef] [PubMed]
- Lazari, G.; Stamatatos, T.C.; Raptopoulou, P.C.; Psycharis, V.; Pissas, M.; Perlepes, S.P.; Boudalis, A.K. A metamagnetic 2D copper(II)-azide complex with 1D ferromagnetism and a hysteretic spin-flop transition. Dalton Trans. 2009, 17, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. Coordination Polymers Containing Manganese(II)-Azido Layers Connected by Dipyridyl-tetrazine and 4,4′-Azobis(pyridine) Linkers. Inorg. Chem. 2013, 52, 1640–1649. [Google Scholar] [CrossRef]
- Cortes, R.; Urtiaga, K.; Lezama, L.; Pizarro, J.L.; Goni, A.; Arriortua, M.I.; Rojo, T. Crystal Structure and Spectroscopic and Magnetic Properties of Two cis-Azido Catenas of Nickel (II): Cis-catena-(μ-N3)[Ni(bipy)2](X)(X= ClO4, PF6). Inorg. Chem. 1994, 33, 4009–4015. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Lloret, F.; Cano, J.; Julve, M. Towards a better understanding of honeycomb alternating magnetic networks. Dalton Trans. 2015, 44, 11040–11051. [Google Scholar] [CrossRef] [PubMed]
- Maalej, W.; Vilminot, S.; Andre, G.; Kurmoo, M. Synthesis, magnetic structure, and properties of a layered cobalt-hydroxide ferromagnet, Co5(OH)6(SeO4)2(H2O)4. Inorg. Chem. 2010, 49, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Lee, G.-H.; Ng, K.K.; Yang, C.-I. A Three-Dimensional Coordination Framework with a Ferromagnetic Coupled Ni(II)-CrO4 Layer: Synthesis, Structure, and Magnetic Studies. Polymers 2022, 14, 1735. [Google Scholar] [CrossRef]
- Dong, S.-S.; Yang, C.-I. A Three-Dimensional Nickel (II) Framework from a Semi-Flexible Bipyrimidyl Ligand Showing Weak Ferromagnetic Behavior. Polymers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-I.; Zhang, Z.-Z.; Lee, G.-H. The use of a semi-flexible bipyrimidyl ligand for the construction of azide-based coordination polymers: Structural diversities and magnetic properties. Dalton Trans. 2018, 47, 16709–16722. [Google Scholar]
- Yang, C.-I.; Zhang, Z.-Z.; Chang, H.-T.; Kuo, Y.L.; Lee, G.-H. Two New Three-Dimensional Pillared-Layer Co(II) and Cu(II) Frameworks Involving a [M2(EO-N3)2] Motif from a Semi-Flexible N-Donor Ligand, 5,5′-Bipyrimidin: Syntheses, Structures and Magnetic Properties. Polymers 2018, 10, 229–243. [Google Scholar]
- Zeng, Y.F.; Hu, X.; Liu, F.C.; Bu, X.H. Azido-mediated systems showing different magnetic behaviors. Chem. Soc. Rev. 2009, 38, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.H.; Jin, X.; Fei, J.S.; Xue, Z.; Li, J.; Tao, L. Spin-canting and weak ferromagnetism in two novel 1D alternating chains with single cis-end-to-end azido bridges. Sci. China Chem. 2013, 56, 656–663. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Mautner, F.A.; Vicente, R. 1D and 2D End-to-End Azide-Bridged Cobalt(II) Complexes: Syntheses, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2007, 46, 4654–4659. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, J.; Yang, N.; Koh, E.K.; Park, J.G.; Hong, C.S. Uncommon ferromagnetic interactions in a homometallic Co2+ chain bridged by a single end-to-end azide. Inorg. Chem. 2007, 46, 9054–9056. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.E. Magnetism in One-Dimensional Systems—The Heisenberg Model for Infinite Spin. Am. J. Phy. 1964, 32, 343–346. [Google Scholar] [CrossRef]
- Hernández, M.L.; Barandika, M.G.; Urtiaga, M.K.; Cortés, R.; Lezama, L.; Arriortua, M.I. Structural analysis and magnetic properties of the 2-D compounds [M(N3)2(bpa)]n (M = Mn, Co or Ni; bpa = 1,2-bis(4- pyridyl)-ethane). J. Chem. Soc. Dalton Trans. 2000, 79–84. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, L.; Wang, Z.M.; Su, G.; Gao, S. Coexistence of spin-canting, metamagnetism, and spin-flop in a (4,4) layered manganese azide polymer. Chem. Mater. 2005, 17, 6369–6380. [Google Scholar] [CrossRef]
- Hong, C.S.; Koo, J.; Son, S.K.; Lee, Y.S.; Kim, Y.S.; Do, Y. Unusual ferromagnetic couplings in single end-to-end azide-bridged cobalt(II) and nickel(II) chain systems. Chem. Eur. J. 2001, 7, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Metropolis, M.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Li, R.-Y.; Wang, X.-Y.; Liu, T.; Xu, H.-B.; Zhao, F.; Wang, Z.-M.; Gao, S. Synthesis, structure, and magnetism of three azido-bridged Co2+ compounds with a flexible coligand 1,2-(tetrazole-1-yl)ethane. Inorg. Chem. 2008, 47, 8134–8142. [Google Scholar] [CrossRef]
- Jiang, Y.-C.; Wang, S.-L.; Lee, S.-F.; Lii, K.-H. Metamagnetism in cobalt phosphates with pillared layer structures: [Co3(pyz)(HPO4)2F2] and [Co3(4,4’-bpy)(HPO4)2F2]xH2O. Inorg. Chem. 2004, 43, 2564–2571. [Google Scholar] [CrossRef]
- Shao, D.; Zhou, Y.; Pi, Q.; Shen, F.-X.; Yang, S.-R.; Zhang, S.-L.; Wang, X.-Y. Two-dimensional frameworks formed by pentagonal bipyramidal cobalt(II) ions and hexacyanometallates: Antiferromagnetic ordering, metamagnetism and slow magnetic relaxation. Dalton Trans. 2017, 46, 9088–9096. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-Y.; Shi, W.; Gao, H.-L.; Cui, J.-Z.; Cheng, P. Spin canting and metamagnetism in 3D pillared-layer homospin cobalt(II) molecular magnetic materials constructed via a mixed ligands approach. Inorg. Chem. Front. 2014, 1, 242–248. [Google Scholar] [CrossRef]
- Yang, B.-P.; Prosvirin, A.V.; Guo, Y.-Q.; Mao, J.-G. Co[HO2C(CH2)3NH(CH2PO3H)2]2: A New Canted Antiferromagnet. Inorg. Chem. 2008, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Balanda, M.; Falk, K.; Griesar, K.; Tomkowicz, Z.; Haase, W. Characterization of magnetic ordering in porphyrin-based molecular magnets [Mn(R)4TPP][TCNE] (R = OC12H25, F. CN). J. Magn. Magn. Mater. 1999, 205, 14–26. [Google Scholar] [CrossRef]
- Cheng, X.-N.; Xue, W.; Zhang, W.-X.; Chen, X.-M. Weak Ferromagnetism and Dynamic Magnetic Behavior of Two 2D Compounds with Hydroxy/Carboxylate-Bridged Co(II) Chains. Chem. Mater. 2008, 20, 5345–5350. [Google Scholar] [CrossRef]
- Li, Z.-X.; Jie, W.; Zha, G.; Wang, T.; Xu, Y. Nitrate-Templated 1D EE-Azide-Cobalt Chain Exhibits Canted Antiferromagnetism and Slow Magnetic Relaxation. Eur. J. Inorg. Chem. 2012, 12, 3537–3540. [Google Scholar] [CrossRef]
- Sheldrick, G.M.J. Appl. Cryst.; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- Sheldrick, G.M. SHELXL2018; University of Goëttingen: Goëttingen, Germany, 2018. [Google Scholar]
- Mulay, L.N.; Boudreaux, E.A. Theory and Applications of Molecular Diamagnetism; Wiley-VCH: New York, NY, USA, 1976; pp. 491–494. ISBN 10:047162358X. [Google Scholar]







| Formula | C14H16CoN8O6 |
|---|---|
| Fw | 451.28 |
| Crystal system | Monoclinic |
| Space group | Cc |
| a/Å | 14.0163(4) |
| b/Å | 6.0132(2) |
| c/Å | 20.9136(6) |
| α/° | 90 |
| β/° | 91.6740(10) |
| γ/° | 90 |
| V/Å3 | 1761.90(9) |
| Z | 4 |
| T/K | 110(2) |
| Dc/g cm−3 | 1.701 |
| μ/mm−1 | 1.029 |
| Rint | 0.0241 |
| θmax/° | 34.983 |
| Goodness-of-fits on F2 | 1.030 |
| R11, wR22 (all data) | 0.0257, 0.0565 |
| R11, wR22 (I > 2σ (I)) | 0.0260, 0.0566 |
| Flack | 0.490(14) |
| Compound 1 | |||
|---|---|---|---|
| Co(1)–O(2) | 2.061(2) | Co(1)–N(7) | 2.111(3) |
| Co(1)–O(1) | 2.085(2) | Co(1)–N(1) | 2.152(3) |
| Co(1)–N(5) | 2.103(2) | Co(1)–N(4) | 2.162(3) |
| O(2)–Co(1)–O(1) | 178.81(10) | N(5)–Co(1)–N(1) | 94.87(10) |
| O(2)–Co(1)–N(5) | 87.42(10) | N(7)–Co(1)–N(1) | 87.13(11) |
| O(1)–Co(1)–N(5) | 91.46(10) | O(2)–Co(1)–N(4) | 92.35(9) |
| O(2)–Co(1)–N(7) | 91.57(11) | O(1)–Co(1)–N(4) | 87.17(10) |
| O(1)–Co(1)–N(7) | 89.53(11) | N(5)–Co(1)–N(4) | 86.35(10) |
| N(5)–Co(1)–N(7) | 177.75(12) | N(7)–Co(1)–N(4) | 91.69(10) |
| O(2)–Co(1)–N(1) | 89.56(10) | N(1)–Co(1)–N(4) | 177.78(12) |
| O(1)–Co(1)–N(1) | 90.95(9) | N(6) 1–N(7)–Co(1) | 132.9(3) |
| N(6)–N(5)–Co(1) | 129.2(3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-L.; Liu, H.-K.; Yang, C.-I. Spin Canting and Weak Ferromagnetism in a New 2D Coordination Polymer with the Co(II) Chain Bridged by a Single End-to-End Azide. Inorganics 2023, 11, 444. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110444
Kuo Y-L, Liu H-K, Yang C-I. Spin Canting and Weak Ferromagnetism in a New 2D Coordination Polymer with the Co(II) Chain Bridged by a Single End-to-End Azide. Inorganics. 2023; 11(11):444. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110444
Chicago/Turabian StyleKuo, Yi-Lin, Hsin-Kuan Liu, and Chen-I Yang. 2023. "Spin Canting and Weak Ferromagnetism in a New 2D Coordination Polymer with the Co(II) Chain Bridged by a Single End-to-End Azide" Inorganics 11, no. 11: 444. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110444