1. Introduction
Over the recent few decades, there has been a growing demand for the development of two-dimensional (2D) materials for energy applications, which has led to the exploration of various metal chalcogenides, driven by their exceptional properties, including high electrical conductivity and stable thermal conductivity [
1,
2]. Among them, metal tellurides are gaining popularity owing to their rich variation in terms of structural and electronic properties. In particular, MoTe
2 [
3,
4] and WTe
2 [
5] have been extensively studied because of their diverse chemical and physical properties and wide range of potential applications in nanotechnology and nanoscience. Despite extensive research on various metal tellurides, noble metal tellurides have not received adequate attention. However, the focus on noble metal tellurides, such as silver telluride and gold telluride, is increasing owing to their promising applications in different domains [
6,
7]. Silver telluride and gold telluride showcase favorable thermoelectric properties, such as a high Seebeck coefficient, low electrical resistivity and low thermal conductivity. Notably, silver telluride has demonstrated excellent thermoelectric properties [
8,
9], while gold telluride has shown potential in thermoelectric applications [
6]. In the superconductivity domain, it is reported that gold telluride undergoes a structural phase transition under high pressure, inducing superconductivity [
10]. Platinum and palladium doping of gold telluride also leads to dimer-breaking-induced superconductivity [
11]. Moreover, silver telluride, which acting as a three dimensional topological insulator, exhibits insulating properties in its bulk form while possessing metallic Dirac fermions on its surface [
12]. Further, silver telluride is an effective catalyst in lithium–oxygen batteries, demonstrating its versatility in catalytic applications [
13].
In recent years, silver (Ag) telluride denoted as Ag
2Te has become increasingly important because of its high electron mobility and low thermal conductivity, making it a desirable candidate for thermoelectric materials [
9,
14,
15]. Ag
2Te displays a low-temperature monoclinic β-Ag
2Te phase featuring semiconductor characteristics with a low-bandgap 0.09 eV at room temperature [
16], and a high-temperature cubic α-Ag
2Te phase with metallic properties, undergoing a transition at 145 °C [
17]. There are different reports on the synthesis of various one-dimension structures of Ag
2Te like nanowires, nanotubes and nanorods [
16,
18,
19]; however, the large-scale growth of ultrathin films based on vapor deposition methods has rarely been reported.
Another noble metal telluride, gold (Au) ditelluride (AuTe
2), has also witnessed limited investigation. AuTe
2 is the only admitted compound in the Au-Te phase diagram [
20] and is naturally found as a mineral calaverite, the only compound in ores that is considered to extract Au from, at the industrial scale [
21]. Despite many interesting features of AuTe
2, such as thermoelectricity [
6] and superconductivity [
11,
22], there are very few reports on its synthesis [
23,
24]. Moreover, there are almost no reports on AuTe
2 chemical vapor deposition (CVD) growth on a large-scale. Recently, 2D Te growth on Au (111) substrates using molecular beam epitaxy has been reported, where the possible scenarios occurring between Te and Au, including the formation of compound AuTe
2, toward the formation of monolayer tellurene on the Au substrate are discussed [
25].
This study provides a comprehensive exploration into large-scale CVD tellurization of single-crystalline Ag (111)/Mica substrate and various Au substrates ranging from polycrystalline Au (50 nm)/SiO2/Si to single-crystalline Au (111)/Mica in order to achieve silver and gold tellurides ultrathin films, respectively. Furthermore, an investigation into the impact of varying the thickness of pre-deposited Au on SiO2/Si from 50 nm down to 10 nm and 5 nm is performed. We studied the chemical, structural and morphological properties of the grown materials via X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM) and Raman spectroscopy analysis.
2. Results and Discussions
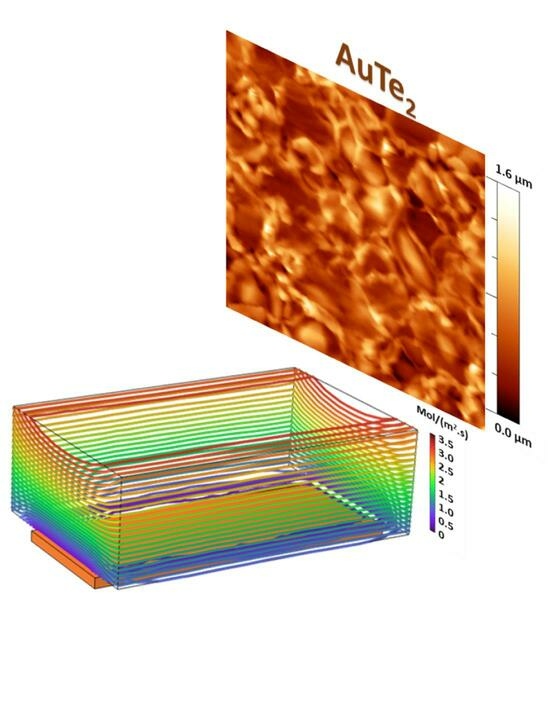
Aiming at studying the silver telluride and gold ditelluride formation based on the tellurization of pre-deposited silver and gold substrates, an AP-CVD system is used.
Figure 1a shows the experimental set-up exploited for the growth, consisting of the double-furnace CVD system detailed in the
Section 3. Among the key thermodynamic parameters for controlling the tellurization of metallic substrates, we can mention gas flux and temperature during the reaction. Given the kinetic character of the tellurization process, the gas flux is expected to play a significant role in increasing the number of sites for tellurium reaction with the metallic atoms of the substrate. This is supported by finite element method (FEM) simulation used as a guide to optimize the experimental condition (see
Section 3 for details). In our experimental setup, typical gas flux values range from 1 sccm to 100 sccm. However, due to the expected low-velocity tellurization reaction in analogy with the sulfurization reaction of metal films [
26], we decided to study the condition of the precursor flux magnitude in the range of 1–10 sccm, excluding the highest flux conditions (>10 sccm), which would result in a low residence time of tellurium vapors in the proximity of the metal precursor films. By mapping the FEM contour lines, connecting the points with equal total flux magnitude, we observed a pronounced increment of the contour levels in proximity of the substrate as the gas flux varied from 1 sccm to 100 sccm (see
Figure S1a–c in Supplementary Materials). The lower flux magnitude is described by far spacing blue lines in
Figure S1a and the higher flux magnitude is represented by more dense red lines in
Figure S1b,c. The quantitative comparison of the total flux magnitude across an arbitrary line over the substrate is depicted in
Figure S1d. The arbitrary line has a length of 3 cm (equal to the length of the substrate). The y-axis shows the total precursor flux magnitude or, in other terms, the amount of the precursor that is reaching the substrate per unit area per unit time (mol/(m
2s)). From the comparison, it is clear that the flux magnitude in the case of 10 sccm carrier gas flow is at least four times higher compared to the 1 sccm flow rate. Although the simulation results reveal a higher precursor flux magnitude over the substrate volume at the carrier gas flux of 100 sccm (
Figure S1c), it is not proven to be optimal for the reaction. This is due to the fact that the increase in the carrier gas flux will lead to the reduction in the residence time of the precursor at the vicinity of the substrate. As a conclusion, the FEM simulation clearly indicate that the optimal experimental conditions for the tellurization reaction are obtained at 10 sccm.
According to the literature, the reaction in bulk form between silver and tellurium occurs at 475 °C [
24], while the reaction between gold and tellurium is reported at 300–500 °C [
27]. In the case of thin films, we studied the growth in the temperature range of 100–350 °C for the downstream furnace under an isobaric condition (pressure of 1 atm/760 torr) and with a 10 sccm Ar/H
2 flux (H
2 4% vol.) as a carrier gas.
In the first set of experiments, the tellurization of the single-oriented Ag(111)/Mica at 100 °C is studied. The Raman spectra acquired on the tellurized sample is illustrated in
Figure 2a and exhibits 7 vibrational modes. According to the literature [
17,
28], the surface of silver chalcogenides is soft and sensitive to laser power. Consequently, there will be a redox reaction upon the exposure of the laser to the sample surface, which may lead to the decomposition of the silver telluride and the segregation of TeO
2 and metallic silver [
17,
28]. To avoid this issue, we acquired the Raman spectra at the lowest power achievable in our setup (1 mW). The broad spectra acquired on the sample are represented in
Figure 2a. The spectra can be decomposed in different spectral components located at 71.0, 91.0, 128.5, 186.3 cm
−1, 236.3 cm
−1, 349.1 cm
−1 and 462.0 cm
−1. The peaks located at 91.0 and 128.5 are raised from in-plane E
1 and out-of-plane A
1 characteristics peaks of Te. The broad peaks at 186.3, 236.3, 349.1 and 462.0 cm
−1 are assigned to TeO
2 [
28,
29,
30]. The observation of peaks related to the TeO
2 may suggest the decomposition of Ag
2Te, according to the following reaction: Ag
2Te + O
2 → 2Ag + TeO
2 [
17,
28]. This leads to the measurement of the Raman peaks of TeO
2. Nevertheless, on the local scale, the AFM morphology investigation shows a compact structure of the film. This is confirmed by the topography image acquired on a 2 μm × 2 μm scan area and depicted in
Figure 2b, showing the closely packed formation of film with rms roughness value, σ, of 20.1 nm. As demonstrated by the silver and tellurium phase diagram of the reaction [
31], the number of possible combinations of the two materials is higher than that of gold and tellurium. Ag
2Te occurs in nature as the mineral hessite and can exist in at least three forms. The stoichiometry Ag
5Te
3 occurs in a mineral called stuetzite, while the metastable phases AgTe, AgTe
4 and AgTe
2.
33 are reported after a very rapid quenching of the molten silver–tellurium alloy [
31].
As a result, the control of the reaction product in the CVD system is more complicated in the case of silver tellurization experiments rather than the gold ones. We have investigated the effect of temperature on the growth of silver telluride by changing the temperature from 100 °C to 350 °C while maintaining all the other experimental parameters. According to the literature [
28], Raman spectroscopy performed on the sample grown at 350 °C has a different characteristic and shows the formation of a peak at 152 cm
−1 (see
Figure 2c) that is compatible with the reported spectra of Ag
5Te
3, which is another compound that exists in the Ag-Te phase diagram. The XPS analysis reported in
Figure S2 confirms the chemical coordination between the Ag 3d and Te 3d core levels. This is supported by the fact that the 3d doublet peaks of Te are located at different binding energies (Te 3d
5/2~572.2 eV, Te 3d
3/2~582.6 eV) compared to the pure elemental state (note that the presence of oxide components is demonstrated by the asymmetry of the spectra). The AFM morphology investigation performed on the 2 μm × 2 μm scan area is represented in
Figure 2d and shows the formation of faceted and elongated structures with an RMS roughness of 22.6 nm.
Following the investigation into the tellurization of the Ag substrate on a large scale, the goal is to explore the use of Au as a pre-deposited substrate. The aim is to examine the impact of transitioning from the polycrystalline Au (50 nm)/SiO
2 substrate to a single-crystalline Au (111)/Mica and also reducing the thickness of Au/SiO
2 from 50 nm down to 10 nm and 5 nm. According to the literature, AuTe
2 was synthesized using an aerosol-assisted CVD technique at temperatures around 300–500 °C [
27]. It has been shown that samples grown at 300 °C exclusively form monoclinic AuTe
2, whereas samples grown at higher temperatures produce Au particles as well, due to the depletion of Te [
27]. The tellurization of various Au substrates is carried out under the same experimental conditions as those exploited for the tellurization of the Ag substrate, utilizing the growth temperature of 350 °C (the effect of a slight change of the temperature condition is described in the supporting information file—see
Figure S3).
The chemical composition of the grown material is investigated by XPS measurement. The high-resolution Au 4f and Te 3d core levels as a function of binding energy (B.E.) are shown in
Figure 3a,b. The experimental data are fitted with a pseudo-Voigt function after background removal. The 4f
7/2 and 4f
5/2 doublets of Au are located at a higher binding energy (B.E. ~84.8 eV and 88.5 eV, respectively) compared to the pure metallic Au (84 eV and 87.6 eV) [
32]; we rationalize this shift of the B.E. with a chemical coordination of the Au atoms with Te atoms in the AuTe
2 formation. The Te 3d broad core-level peaks are fitted by two components, where the binding energy of most important peaks, Te 3d
5/2 and 3d
3/2 (B.E. ~573.2 eV and 583.6 eV, respectively), are related to pure elemental tellurium [
11]. The components located at ~576.6 and ~587 eV for 3d
5/2 and 3d
3/2 can be related to Te oxide due to the exposure to air before the XPS measurement.
To study the crystal structure of the sample, we used XRD analysis. The acquired XRD pattern with the sketch of the crystal structure acquired by Avogadro-1.2.0 software are depicted in
Figure 3c. The results show the existence of two main peaks located at nearly 29° and 42.9° relating to the [−1,1,1] and [3,1,0] crystallographic orientation, in addition to few broad peaks matching with the AuTe
2 reference pattern [
33]. Since AuTe
2 exhibits an incommensurately modulated structure, the XRD pattern shows satellites along with main reflections [
34]. Therefore, the coordination of gold atoms changes from linear to square planar due to modulation, and the Te zig-zag chains are broken, resulting in isolated Te-Te pairs [
32,
33]. This average phase has a distorted CdI2 structure,with each Au atom being sixfold coordinated with Te atoms and two long and four short Au-Te bonds. The AuTe6 octahedra form layers parallel to the (001) plane as shown in the inset of
Figure 3c [
35].
According to the literature [
36], the incommensurate modulation of the AuTe
2 crystal structure has a strong influence on its optical and vibrational spectrum. In particular, it is responsible for the optical selection rules allowing Raman scattering of phonons with a wavevector q ≠ 0 [
36]. As a result, the Raman spectra of the sample after the fitting by the Voigt function and subtraction of the background (due to large background contribution coming from the gold substrate) shows more peaks than expected for a crystal with three atoms per primitive cells [
36]. We observed six peaks located at 106.0, 114.1, 123.4, 138.0, 148.0 and 155.7 cm
−1 (see
Figure 3d). The precise assignment of the peaks to a vibrational mode is not straightforward because of the expected degeneracy of the modes belonging to the different symmetries: A
g, A
u and B
g [
36]. It is worth to notice that the intensity ratio of the first peaks (located at 106.0 cm
−1) with respect to the last peaks (located at 155.7 cm
−1) undergoes a variation. In detail, it increases from 0.8 to 1.7, while moving from the region of the sample closer to the tellurium source (named “start point” in plot of
Figure 3d) towards the farther region (named “end point” in plot of
Figure 3d). On the one hand, we can rationalize this observation by assuming a reduction of the tellurized thickness of the gold film precursor as a function of the position; on the other hand, this demonstrates that the formation of AuTe
2 occurs on a large scale (sample area ≈ cm
2).
It is interesting to study the morphological features of the grown material at the nanoscale by AFM and make a direct comparison of the morphology of the Au/SiO
2 and Au (111)/Mica substrates before and after tellurization (see
Figure 4 and
Figure S4). As shown in
Figure 4a, the surface morphology of the pre-deposited Au on SiO
2/Si consists of small grains, whereas the surface morphology of Au (111) film is different and shows a uniform surface (see
Figure 4c). The morphology of tellurized Au/SiO
2 and Au(111)/Mica films represented in
Figure 4b,d, respectively, exhibits the formation of textured material characterized by a continuous and completely covered surface being constituted by large grains. The quantitative estimation of the statistical height distribution is provided by the rms roughness value, σ, in the two cases (see
Section 3 The σ variable is derived by analyzing several AFM images (2 µm × 2 µm) acquired at different spatial positions of the sample and by calculating the average value and the statistical standard variation used as uncertainty on the variable. We observe that σ varies from 1.0 ± 0.1 nm in the case of the e-beam deposited Au on SiO
2/Si substrate to 33.8 ± 2 nm for the AuTe
2 sample after tellurization. Similarly, in the case of Au (111)/Mica, σ varies from 2.7 ± 0.1 nm to 23.1 ± 2 nm for AuTe
2 after tellurization. Another statistical parameter derived by our analysis is the mean size of the polycrystalline grains observed in the AFM topographic images. It is worth noting that we applied two different methods for calculating the mean grain size in the topography of
Figure 4a with respect to
Figure 4b,d (in the case of the Au(111) film. Moreover, the size of the crystalline domains exceeds the typical size of the AFM image, as one can notice in the topography of
Figure 4c, where only a small portion of a grain boundaries can be detected in the bottom left part of the map). As noted above, the topography of
Figure 4a shows the presence of round-shaped grains obtained by the evaporation of gold atoms on the substrate surface. In this case, we can apply the analysis of the cross-correlation function of the figure to derive the average size of the grains as the full width at half maximum of the correlation peak (see
Section 3 and
Supplementary Materials for further details). From this analysis, we estimated that for the Au/SiO
2 substrate, the average grain size is around 40 ± 2 nm. This method cannot be applied to the topographies of
Figure 4b,d due to the fact that the grains show a more irregular shape and spatial distribution. For this reason, we identified the areal distribution of the grains in several AFM images by manually defining the contour line of each single grain and performing statistical calculations on the derived distribution. In the two cases, we observed that after tellurization, the grain size of the AuTe
2 reads 870 ± 97 nm (starting from the evaporated gold precursor) and 592 ± 75 nm (starting from the single-crystalline Au(111) film).
The large-scale controlled growth of AuTe
2 starting from polycrystalline Au(50 nm)/SiO
2 and single-crystalline Au(111)/Mica are successfully demonstrated. The findings reveal that despite the different crystalline characteristics of the substrate (polycrystalline or single-crystalline), the morphological properties of AuTe
2 are similar in both cases. The morphological similarity between the tellurized films starting from different substrates (polycrystalline or single-crystalline) demonstrates that the AuTe
2 material does not inherit the morphological features of the metallic precursor film. This fact suggests that the mechanism of tellurization does not proceed at the scale of the single gold grain. We speculate that the reaction can occur in two possible ways: The first one is a reaction of the gold and tellurium atoms in the vapor phase and subsequent deposition on the surface. The second one is a reaction between a liquid layer of gold and tellurium vapors at the surface and subsequent re-crystallization. Moreover, after the large-scale synthesizing process of the uniform AuTe
2 film, the tellurization of Au/SiO
2 substrates with varying thicknesses from 50 nm down to 10 nm and 5 nm will be reported.
Figure S5 illustrates the Raman spectra obtained from AuTe
2 films with 10 nm and 5 nm pre-deposited Au substrate thicknesses. The Raman peaks identified at 105.6, 115.0, 124.1, 138.5, 148.5 and 156.4 cm
−1 (
Figure S5) are aligned with the observed Raman spectra of AuTe
2 derived from Au with the thickness of 50 nm (in the previous section). The AFM topography images acquired on AuTe
2 from Au with the 10 nm and 5 nm thicknesses are represented in
Figure 5a,b and show the formation of compact granular grains with average sizes of 142 ± 14 nm and 78 ± 9 nm, respectively (
Figure S6). The rms roughness value, σ, exhibits variation between different samples. It ranges from 22.2 ± 1.4 nm for the AuTe
2 sample with a 10 nm Au substrate thickness to 10.0 ± 0.6 nm for the AuTe
2 sample with a 5 nm Au substrate thickness.
By comparing the calculated average grain size obtained from different Au/SiO
2 substrate thicknesses (see
Figure 5c), it can be understood that there is a correlation between the initial Au substrate thickness and the resulting grain size of AuTe
2 after the tellurization process. It can be interpreted that reducing the thickness of pre-deposited Au on SiO
2/Si leads to a smaller grain size. This tellurization method is applicable not only to different gold substrates (polycrystalline and single-crystalline) but also to different thicknesses of gold, ranging from 50 nm to 10 nm and 5 nm.