Dielectric Spectroscopy of Non-Stoichiometric SrMnO3 Thin Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Characterization
2.2. Dielectric Characterization
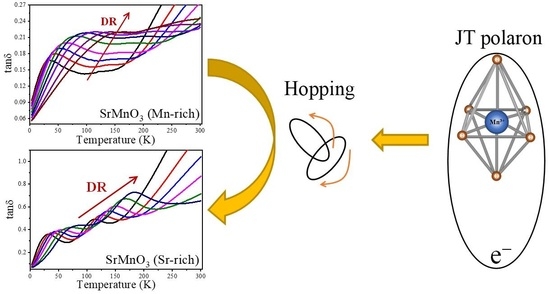
2.3. Relaxation Mechanism Analysis
3. Experimental Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Ke, X.; Misra, R.; Ihlefeld, J.F.; Xu, X.S.; Mei, Z.G.; Heeg, T.; Roeckerath, M.; Schubert, J.; Liu, Z.K.; et al. Adsorption-controlled growth of BiMnO3 films by molecular-beam epitaxy. Appl. Phys. Lett. 2010, 96, 262905. [Google Scholar] [CrossRef]
- Pashchenko, A.V.; Liedienov, N.A.; Li, Q.; Makoed, I.I.; Tatarchuk, D.D.; Didenko, Y.V.; Gudimenko, A.I.; Kladko, V.P.; Jiang, L.; Li, L.; et al. Control of dielectric properties in bismuth ferrite multiferroic by compacting pressure. Mater. Chem. Phys. 2021, 258, 123925. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Goldschmidt, V.V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Zhao, X.N.; Xu, H.Y.; Wang, Z.Q.; Lin, Y.; Liu, Y.C. Memristors with organic-inorganic halide perovskites. InfoMat 2019, 1, 183–210. [Google Scholar] [CrossRef]
- Deng, W.; Jin, X.C.; Lv, Y.; Zhang, X.J.; Zhang, X.H.; Jie, J.S. 2D Ruddlesden–Popper Perovskite Nanoplate Based Deep-Blue Light-Emitting Diodes for Light Communication. Adv. Funct. Mater. 2019, 29, 1903861. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Deng, W.; Huang, L.M.; Xu, X.Z.; Zhang, X.J.; Jin, X.C.; Lee, S.T.; Jie, J.S. Ultrahigh-Responsivity Photodetectors from Perovskite Nanowire Arrays for Sequentially Tunable Spectral Measurement. Nano Lett. 2017, 17, 2482–2489. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Spaldin, N.A. Functional Ion Defects in Transition Metal Oxides. Science 2013, 341, 858–859. [Google Scholar] [CrossRef] [PubMed]
- Malyi, O.I.; Yeung, M.T.; Poeppelmeier, K.R.; Persson, C.; Zunger, A. Spontaneous Non-stoichiometry and Ordering in Degenerate but Gapped Transparent Conductors. Matter 2019, 1, 280–294. [Google Scholar] [CrossRef]
- Bai, J.W.; Yang, J.; Dong, W.X.; Zhang, Y.Y.; Bai, W.; Tang, X.D. Structural and magnetic properties of perovskite SrMnO3 thin films grown by molecular beam epitaxy. Thin Solid Films 2017, 644, 57–64. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.Q.; Bai, Y.; Liu, W.; Zhang, H.R.; Wang, W.Y.; Li, S.K.; Ma, S.; Zhao, X.G.; Sun, J.R.; et al. Oxygen vacancy formation, crystal structures, and magnetic properties of three SrMnO3-δ films. Appl. Phys. Lett. 2016, 109, 052403. [Google Scholar] [CrossRef]
- Maurel, L.; Marcano, N.; Prokscha, T.; Langenberg, E.; Blasco, J.; Guzmán, R.; Suter, A.; Magén, C.; Morellón, L.; Ibarra, M.R.; et al. Nature of antiferromagnetic order in epitaxially strained multiferroic SrMnO3 thin films. Phys. Rev. B 2015, 92, 024419. [Google Scholar] [CrossRef]
- Patrakeev, M.V.; Bahteeva, J.A.; Mitberg, E.B.; Leonidov, I.A.; Kozhevnikov, V.L.; Poeppelmeier, K.R. Electron/hole and ion transport in La1-XSrxFeO3-δ. J. Solid State Chem. 2003, 172, 219–231. [Google Scholar] [CrossRef]
- Casais, M.T.; Alonso, J.A.; Rasines, I.; Hidalgo, M.A. Preparation, neutron structural study and characterization of BaNbO3: A Pauli-like metallic perovskite. Mater. Res. Bull. 1995, 30, 201–208. [Google Scholar] [CrossRef]
- Nadkarni, N.; Zhou, T.T.; Fraggedakis, D.; Gao, T.; Bazant, M.Z. Modeling the Metal–Insulator Phase Transition in LixCoO2 for Energy and Information Storage. Adv. Funct. Mater. 2019, 29, 1902821. [Google Scholar] [CrossRef]
- Li, T.; Deng, S.; Qi, H.; Zhu, T.; Chen, Y.; Wang, H.; Zhu, F.; Liu, H.; Wang, J.; Guo, E.J.; et al. High-Temperature Ferroic Glassy States in SrTiO3-Based Thin Films. Phys. Rev. Lett. 2023, 131, 246801. [Google Scholar] [CrossRef]
- Kan, D.; Orikasa, Y.; Nitta, K.; Tanida, H.; Kurosaki, R.; Nishimura, T.; Sasaki, T.; Guo, H.C.; Ozaki, Y.; Uchimoto, Y.; et al. Overpotential-Induced Introduction of Oxygen Vacancy in La0.67Sr0.33MnO3 Surface and Its Impact on Oxygen Reduction Reaction Catalytic Activity in Alkaline Solution. J. Phys. Chem. C 2016, 120, 6006–6010. [Google Scholar] [CrossRef]
- Islamov, D.R.; Zalyalov, T.M.; Orlov, O.M.; Gritsenko, V.A.; Krasnikov, G.Y. Impact of oxygen vacancy on the ferroelectric properties of lanthanum-doped hafnium oxide. Appl. Phys. Lett. 2020, 117, 162901. [Google Scholar] [CrossRef]
- Trabelsi, H.; Bejar, M.; Dhahri, E.; Sajieddine, M.; Khirouni, K.; Prezas, P.R.; Melo, B.M.G.; Valente, M.A.; Graça, M.P.F. Effect of oxygen vacancies on SrTiO3 electrical properties. J. Alloys Compd. 2017, 723, 894–903. [Google Scholar] [CrossRef]
- Liu, Y.K.; Wong, H.F.; Lam, K.K.; Mak, C.L.; Leung, C.W. Tuning ferromagnetic properties of LaMnO3 films by oxygen vacancies and strain. J. Magn. Magn. Mater. 2019, 481, 85–92. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cho, C.W.; Kim, J.W.; Bae, J.S.; Yun, H.J.; Lee, J.; Park, S.Y. Effect of oxygen vacancies in the magnetic properties of the amorphous CoFe2O4 films. J. Non-Cryst. Solids 2017, 456, 83–87. [Google Scholar] [CrossRef]
- Chen, Y.H.; Luo, D.B.; Cao, X.Y.; Wang, Y.F.; Aung, P.; Jin, K.X.; Wang, S.H. Effect of oxygen vacancies on the electrical transport properties of conductive Y3Fe5O12 films at high temperature. J. Phys. D Appl. Phys. 2023, 56, 455107. [Google Scholar] [CrossRef]
- Zulueta, Y.A.; Dawson, J.A.; Leyet, Y.; Guerrero, F.; Anglada-Rivera, J.; Nguyen, M.T. Influence of titanium and oxygen vacancies on the transport and conducting properties of barium titanate. Phys. Status Solidi B 2016, 253, 345–350. [Google Scholar] [CrossRef]
- Sun, H.Y.; Mao, Z.W.; Zhang, T.W.; Han, L.; Zhang, T.T.; Cai, X.B.; Guo, X.; Li, Y.F.; Zang, Y.P.; Guo, W.; et al. Chemically specific termination control of oxide interfaces via layer-by-layer mean inner potential engineering. Nat. Commun. 2018, 9, 2965. [Google Scholar] [CrossRef]
- Becher, C.; Maurel, L.; Aschauer, U.; Lilienblum, M.; Magen, C.; Meier, D.; Langenberg, E.; Trassin, M.; Blasco, J.; Krug, I.P.; et al. Strain-induced coupling of electrical polarization and structural defects in SrMnO3 films. Nat. Nanotechnol. 2015, 10, 661–665. [Google Scholar] [CrossRef]
- Guo, H.Z.; Wang, J.O.; He, X.; Yang, Z.Z.; Zhang, Q.H.; Jin, K.J.; Ge, C.; Zhao, R.Q.; Gu, L.; Feng, Y.Q.; et al. The Origin of Oxygen Vacancies Controlling La2/3Sr1/3MnO3 Electronic and Magnetic Properties. Adv. Mater. Interfaces 2016, 3, 1500753. [Google Scholar] [CrossRef]
- Lei, Q.Y.; Golalikhani, M.; Davidson, B.; Liu, G.Z.; Schlom, D.G.; Qiao, Q.; Zhu, Y.M.; Chandrasena, R.U.; Yang, W.B.; Gray, A.X.; et al. Constructing oxide interfaces and heterostructures by atomic layer-by-layer laser molecular beam epitaxy. Npj Quantum Mater. 2017, 2, 10. [Google Scholar] [CrossRef]
- Haislmaier, R.C.; Grimley, E.D.; Biegalski, M.D.; LeBeau, J.M.; Trolier-McKinstry, S.; Gopalan, V.; Engel-Herbert, R. Unleashing Strain Induced Ferroelectricity in Complex Oxide Thin Films via Precise Stoichiometry Control. Adv. Funct. Mater. 2016, 26, 7271–7279. [Google Scholar] [CrossRef]
- Jalan, B.; Moetakef, P.; Stemmer, S. Molecular beam epitaxy of SrTiO3 with a growth window. Appl. Phys. Lett. 2009, 95, 032906. [Google Scholar] [CrossRef]
- Ohnishi, T.; Shibuya, K.; Yamamoto, T.; Lippmaa, M. Defects and transport in complex oxide thin films. J. Appl. Phys. 2008, 103, 103703. [Google Scholar] [CrossRef]
- Bai, J.W.; Liu, Q.Q.; Wu, M.; Yang, J.; Jiang, W.; Wang, J.L.; Bai, W.; Zhang, Y.Y.; Tang, X.D.; Chu, J.H. Correlation of oxygen vacancy and Jahn–Teller polarons in epitaxial perovskite SrMnO3 ultrathin films: Dielectric spectroscopy investigations. Appl. Phys. Lett. 2020, 116, 142901. [Google Scholar] [CrossRef]
- Guzman, R.; Maurel, L.; Langenberg, E.; Lupini, A.R.; Algarabel, P.A.; Pardo, J.A.; Magen, C. Polar-Graded Multiferroic SrMnO3 Thin Films. Nano Lett. 2016, 16, 2221–2227. [Google Scholar] [CrossRef]
- Brooks, C.M.; Kourkoutis, L.F.; Heeg, T.; Schubert, J.; Muller, D.A.; Schlom, D.G. Growth of homoepitaxial SrTiO3 thin films by molecularbeam epitaxy. Appl. Phys. Lett. 2009, 94, 162905. [Google Scholar] [CrossRef]
- Tokuda, Y.; Kobayashi, S.; Ohnishi, T.; Mizoguchi, T.; Shibata, N.; Ikuhara, Y.; Yamamoto, T. Strontium vacancy clustering in Ti-excess SrTiO3 thin film. Appl. Phys. Lett. 2011, 99, 033110. [Google Scholar] [CrossRef]
- Chan, N.H.; Sharma, R.K.; Smyth, D.M. Nonstoichiometry in Undoped BaTiO3. J. Am. Ceram. Soc. 1981, 64, 556–562. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Zhong, Q.L.; Bai, J.W.; Yang, J.; Huang, R.; Bai, W.; Zhang, Y.Y.; Duan, C.G.; Tang, X.D. Specific cation stoichiometry control of SrMnO3-δ thin films via RHEED oscillations. Appl. Phys. Lett. 2021, 118, 232903. [Google Scholar] [CrossRef]
- Yang, J.; Bai, W.; Zhang, Y.; Duan, C.G.; Chu, J.; Tang, X. Dielectric phenomena of multiferroic oxides at acoustic- and radio-frequency. J. Phys. Condens. Matter 2023, 35, 463001. [Google Scholar] [CrossRef]
- Maglione, M. Free charge localization and effective dielectric permittivity in oxides. J. Adv. Dielectr. 2016, 06, 1630006. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Loidl, A. Dielectric spectroscopy on organic charge-transfer salts. J. Phys. Condens. Matter 2015, 27, 373001. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Krohns, S.; Riegg, S.; Ebbinghaus, S.G.; Reller, A.; Loidl, A. Colossal dielectric constants in transition-metal oxides. Eur. Phys. J. Spec. Top. 2010, 180, 61–89. [Google Scholar] [CrossRef]
- Ahad, A.; Shukla, D.K.; Rahman, F.; Gautam, K.; Dey, K.; Majid, S.S.; Sharma, S.K.; Coaquira, J.A.H. Griffiths-like phase and charge-spin glass state in La1.5Sr0.5CoO4. Appl. Phys. Lett. 2018, 113, 102405. [Google Scholar] [CrossRef]
- Kagawa, F.; Mochizuki, M.; Onose, Y.; Murakawa, H.; Kaneko, Y.; Furukawa, N.; Tokura, Y. Dynamics of multiferroic domain wall in spin-cycloidal ferroelectric DyMnO3. Phys. Rev. Lett. 2009, 102, 057604. [Google Scholar] [CrossRef]
- Xu, G.; Zhong, Z.; Bing, Y.; Ye, Z.G.; Shirane, G. Electric-field-induced redistribution of polar nano-regions in a relaxor ferroelectric. Nat. Mater. 2006, 5, 134–140. [Google Scholar] [CrossRef]
- Guerra, J.D.L.S.; Lente, M.H.; Eiras, J.A. Microwave dielectric dispersion process in perovskite ferroelectric systems. Appl. Phys. Lett. 2006, 88, 102905. [Google Scholar] [CrossRef]
- Park, T.; Nussinov, Z.; Hazzard, K.R.; Sidorov, V.A.; Balatsky, A.V.; Sarrao, J.L.; Cheong, S.W.; Hundley, M.F.; Lee, J.S.; Jia, Q.X.; et al. Novel dielectric anomaly in the hole-doped La2Cu1-xLixO4 and La2-xSrxNiO4 insulators: Signature of an electronic glassy state. Phys. Rev. Lett. 2005, 94, 017002. [Google Scholar] [CrossRef]
- Chen, A.; Zhi, Y. Oxygen-vacancy-related low-frequency dielectric relaxation and electrical conduction in Bi:SrTiO3. Phys. Rev. B 2000, 62, 228–236. [Google Scholar] [CrossRef]
- Jonscher, A.K. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, 57–70. [Google Scholar] [CrossRef]
- Raistrick, I.D.; Franceschetti, D.R.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Savinov, M.; Bovtun, V.; Tereshina-Chitrova, E.; Stupakov, A.; Dejneka, A.; Tyunina, M. Dielectric relaxation in epitaxial films of paraelectric-magnetic SrTiO3-SrMnO3 solid solution. Appl. Phys. Lett. 2018, 112, 052901. [Google Scholar] [CrossRef]
- Bujakiewicz-Koronskaa, R.; Nalecza, D.M.; Majcherb, A.M.; Juszynska-Galazkac, E.G.M.; Vasylechkod, L.; Markiewicze, E.; Majdaf, D.; Kalvaneg, A.; Koronskih, K. Structural, magnetic, dielectric and mechanical properties of (Ba,Sr)MnO3 ceramics. J. Eur. Ceram. Soc. 2017, 37, 1477–1486. [Google Scholar] [CrossRef]
- May, S.J.; Santos, T.S.; Bhattacharya, A. Onset of metallic behavior in strained (LaNiO3)n/(SrMnO3)2 superlattices. Phys. Rev. B 2009, 79, 115127. [Google Scholar] [CrossRef]
- Imada, M.; Fujimori, A.; Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 1998, 70, 1039–1263. [Google Scholar] [CrossRef]
- Edwardst, S.F.; Anderson, P.W. Theory of spin glasses. J. Phys. F Met. Phys. 1975, 5, 965–974. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Yang, J.; Liu, Q.; Bai, J.; Bai, W.; Zhang, Y.; Tang, X. Dielectric Spectroscopy of Non-Stoichiometric SrMnO3 Thin Films. Inorganics 2024, 12, 71. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics12030071
Zeng S, Yang J, Liu Q, Bai J, Bai W, Zhang Y, Tang X. Dielectric Spectroscopy of Non-Stoichiometric SrMnO3 Thin Films. Inorganics. 2024; 12(3):71. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics12030071
Chicago/Turabian StyleZeng, Shuang, Jing Yang, Qingqing Liu, Jiawei Bai, Wei Bai, Yuanyuan Zhang, and Xiaodong Tang. 2024. "Dielectric Spectroscopy of Non-Stoichiometric SrMnO3 Thin Films" Inorganics 12, no. 3: 71. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics12030071