Synthesis of Trithia-Borinane Complexes Stabilized in Diruthenium Core: [(Cp*Ru)2(η1-S)(η1-CS){(CH2)2S3BR}] (R = H or SMe)
Abstract
:1. Introduction
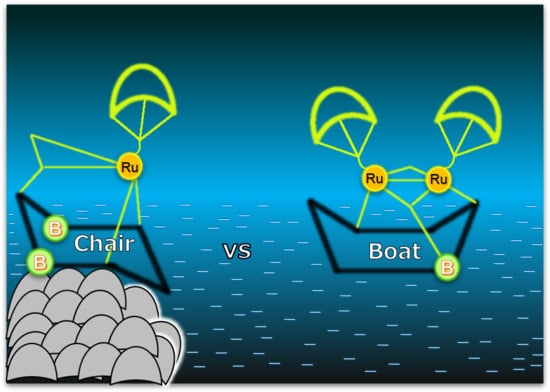
2. Results and Discussion
Synthesis of Ruthenium Borinane Complexes, 2–4
3. Materials and Methods
3.1. General Procedures and Instrumentation
3.2. Synthesis
3.2.1. Synthesis of Compounds 2, 3, and 4
3.2.2. Synthesis of Compound 5
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartwig, J.F.; Muhoro, C.N.; He, X.; Eisenstein, O.; Bosque, R.; Maseras, F. Catecholborane Bound to Titanocene. Unusual Coordination of Ligand σ-Bonds. J. Am. Chem. Soc. 1996, 118, 10936–10937. [Google Scholar] [CrossRef]
- Douglas, T.M.; Chaplin, A.B.; Weller, A.S. Amine–Borane σ-Complexes of Rhodium. Relevance to the Catalytic Dehydrogenation of Amine–Boranes. J. Am. Chem. Soc. 2008, 130, 14432–14433. [Google Scholar] [CrossRef] [PubMed]
- Forster, D.; Tuononen, H.M.; Parvez, M.; Roesler, R. Characterization of β-B-Agostic Isomers in Zirconocene Amidoborane Complexes. J. Am. Chem. Soc. 2009, 131, 6689–6691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.Y.; Thompson, A.L.; Aldridge, S. Rhodium and Iridium Aminoborane Complexes: Coordination Chemistry of BN Alkene Analogues. Angew. Chem. 2010, 122, 933–937. [Google Scholar] [CrossRef]
- Crossley, I.R.; Foreman, M.R.S.J.; Hill, A.F.; White, A.J.P.; Williams, D.J. The first rhodaboratrane: [RhCl(PPh3){B(mt)3}](Rh→B) (mt = methimazolyl). Chem. Commun. 2005, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lei, X.; Shang, M.; Fehlner, T.P. Role of the Transition Metal in Metallaborane Chemistry. Reactivity of (Cp*ReH2)2B4H4 with BH3·thf, CO, and Co2(CO)8. Inorg. Chem. 2000, 39, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Ward, I.M. Metalloboranes and metal–boron bonding. Chem. Soc. Rev. 1974, 3, 231–271. [Google Scholar] [CrossRef]
- Grimes, R.N. Structure and stereochemistry in metalloboron cage compounds. Acc. Chem. Res. 1978, 11, 420–427. [Google Scholar] [CrossRef]
- Fehlner, T.P. A molecular orbital analysis of four chromaboranes: On the curious behavior of (η5-C5R5)Cr fragments in a borane cluster environment. J. Organomet. Chem. 1998, 550, 21. [Google Scholar] [CrossRef]
- Ghosh, S.; Beatty, A.M.; Fehlner, T.P. The Reaction of Cp*ReH6, Cp* = C5Me5 with Monoborane to Yield a Novel Rhenaborane. Synthesis and Characterization of arachno-Cp*ReH3B3H8. Collect. Czech. Chem. Commun. 2002, 67, 808–812. [Google Scholar] [CrossRef]
- Sahoo, S.; Reddy, K.H.K.; Dhayal, R.S.; Mobin, S.M.; Jemmis, E.D.; Ghosh, S. Chlorinated Hypoelectronic Dimetallaborane Clusters Synthesis, Characterization, Electronic Structures of (η5-Cp*W)2B5HnClm (n = 7, m = 2; n = 8, m = 1). Inorg. Chem. 2009, 48, 6509–6516. [Google Scholar] [CrossRef] [PubMed]
- Dhayal, R.S.; Sahoo, S.; Reddy, K.H.K.; Mobin, S.M.; Jemmis, E.D.; Ghosh, S. Vertex-Fused Metallaborane Clusters: Synthesis, Characterization and Electronic Structure of [(η5-C5Me5Mo)3MoB9H18]. Inorg. Chem. 2010, 49, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Noll, B.C.; Fehlner, T.P. Expansion of Iridaborane Clusters by Addition of Monoborane. Novel Metallaboranes and Mechanistic Detail. Dalton Trans. 2008, 371–378. [Google Scholar] [CrossRef]
- Geetharani, K.; Krishnamoorthy, B.S.; Kahlal, S.; Mobin, S.M.; Halet, J.-F.; Ghosh, S. Synthesis and Characterization of Tantalaboranes. Comparison of the Geometric and Electronic Structures of [(Cp*TaX)2B5H11] (X = Cl, Br and I). Inorg. Chem. 2012, 51, 10176–10184. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Noll, B.C.; Fehlner, T.P. Borane Mimics of Classic Organometallic Compounds: [(Cp*Ru)(B8H14)(RuCp*)]0,+1 Isoelectronic Analogues of Dinuclear Pentalene Complexes. Angew. Chem. Int. Ed. 2005, 44, 6568–6571. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Fehlner, T.P. Triborane. A transition metal ligand or heterocluster fragment? Inorg. Chem. 1982, 21, 1739. [Google Scholar] [CrossRef]
- Housecroft, C.E. Boranes and Metallaboranes; Ellis Horwood: Chichester, UK, 1990. [Google Scholar]
- Mingos, D.M.P. Inorganometallic Chemistry; Fehlner, T.P., Ed.; Plenum: New York, NY, USA, 1992. [Google Scholar]
- Hoffmann, R. Building Bridges Between Inorganic and Organic Chemistry (Nobel Lecture). Angew. Chem. Int. Ed. 1982, 21, 711–724. [Google Scholar] [CrossRef]
- Chakrahari, K.K.V.; Dudekula, S.; Barik, S.K.; Mondal, B.; Varghese, B.; Ghosh, S. Hypoelectronic Metallaboranes: Synthesis, Structural Characterization, and Electronic Structures of the Metal-Rich Cobaltaboranes. J. Organomet. Chem. 2014, 749, 188–196. [Google Scholar] [CrossRef]
- Geetharani, K.; Bose, S.K.; Pramanik, G.; Saha, T.K.; Ramkumar, V.; Ghosh, S. An Efficient Route to Group 6 and 8 Metallaborane Compounds: Synthesis of arachno-[Cp*Fe(CO)B3H8] and closo-[(Cp*M)2B5H9] (M = Mo, W). Eur. J. Inorg. Chem. 2009, 1483–1487. [Google Scholar] [CrossRef]
- Roy, D.K.; Mondal, B.; Shankhari, P.; Anju, R.S.; Geetharani, K.; Mobin, S.M.; Ghosh, S. Supraicosahedral Polyhedra: Synthesis and Structural Characterization of 12, 15 and 16-vertex Rhoda-boranes. Inorg. Chem. 2013, 52, 6705–6712. [Google Scholar] [CrossRef]
- Geetharani, K.; Bose, S.K.; Sahoo, S.; Mobin, S.M.; Ghosh, S. Cluster Expansion Reactions of Group 6 and 8 Metallaboranes Using Transition Metal Carbonyl Compounds of Gr 7-9. Inorg. Chem. 2011, 50, 5824–5832. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.K.; Bose, S.K.; Anju, R.S.; Ramkumar, V.; Ghosh, S. Synthesis and Structure of Dirhodium Analogue of Octaborane-12 and Decaborane-14. Inorg. Chem. 2012, 51, 10715–10722. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Geetharani, K.; Sahoo, S.; Reddy, K.H.K.; Varghese, B.; Jemmis, E.D.; Ghosh, S. Synthesis, Characterization, and Electronic Structure of New Type of Heterometallic Boride Clusters. Inorg. Chem. 2011, 50, 9414–9422. [Google Scholar] [CrossRef] [PubMed]
- Anju, R.S.; Roy, D.K.; Mondal, B.; Yuvaraj, K.; Arivazhagan, C.; Saha, K.; Varghese, B.; Ghosh, S. Reactivity of Diruthenium and Dirhodium Analogues of Pentaborane(9): Agostic versus Boratrane Complexes. Angew. Chem. Int. Ed. 2014, 53, 2873–2877. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Ramalakshmi, R.; Gomosta, S.; Pathak, K.; Dorcet, V.; Roisnel, T.; Halet, J.-F.; Ghosh, S. Design, Synthesis, and Chemistry of Bis(σ)borate and Agostic Complexes of Group 7 Metals. Chem. Eur. J. 2017, 23, 9812–9820. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Joseph, B.; Borthakur, R.; Ramalakshmi, R.; Roisnel, T.; Ghosh, S. Chemistry of ruthenium σ-borane complex, [Cp*RuCO(μ-H)BH2L] (Cp* = η5-C5Me5; L = C7H4NS2) with terminal and internal alkynes: Structural characterization of vinyl hydroborate and vinyl complexes of ruthenium. Polyhedron 2017, 125, 246–252. [Google Scholar] [CrossRef]
- Roy, D.K.; Borthakur, R.; De, A.; Varghese, B.; Phukan, A.K.; Ghosh, S. Synthesis and Characterization of Bis(sigma)borate and Bis-zwitterionic Complexes of Rhodium and Iridium. ChemistrySelect 2016, 1, 3757–3761. [Google Scholar] [CrossRef]
- Anju, R.S.; Mondal, B.; Saha, K.; Panja, S.; Varghese, B.; Ghosh, S. Hydroboration of Alkynes with Zwitterionic Ruthenium–Borate Complexes: Novel Vinylborane Complexes. Chem. Eur. J. 2015, 21, 11393–11400. [Google Scholar] [CrossRef]
- Ramalakshmi, R.; Saha, K.; Roy, D.K.; Varghese, B.; Phukan, A.K.; Ghosh, S. New Routes to a Series of σ-Borane/Borate Complexes of Molybdenum and Ruthenium. Chem. Eur. J. 2015, 21, 17191–17195. [Google Scholar] [CrossRef]
- Anju, R.S.; Roy, D.K.; Geetharani, K.; Mondal, B.; Varghese, B.; Ghosh, S. A fine tuning of metallaborane to bridged-boryl complex, [(Cp*Ru)2(μ-H)(μ-CO)(μ-Bcat)] (cat = 1,2-O2C6H4; Cp* = η5-C5Me5). Dalton Trans. 2013, 42, 12828–12831. [Google Scholar] [CrossRef]
- Saha, K.; Kaur, U.; Kar, S.; Mondal, B.; Joseph, B.; Antharjanam, P.K.S.; Ghosh, S. Trithia-diborinane and Bis(bridging-boryl) Complexes of Ruthenium Derived from a [BH3(SCHS)]− Ion. Inorg. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, D.; Yuvaraj, K.; Barik, S.K.; Roy, D.K.; Chakrahari, K.K.; Ramalakshmi, R.; Mondal, B.; Varghese, B.; Ghosh, S. New Heteronuclear Bridged Borylene Complexes That Were Derived from [{Cp*CoCl}2] and Mono-Metal–Carbonyl Fragments. Chem. Eur. J. 2013, 19, 15219–15225. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Prakash, R.; Jagan, R.; Ghosh, S. Synthesis and ligand substitution of tri-metallic triply bridging borylene complexes. J. Organomet. Chem. 2018, 866, 79–86. [Google Scholar] [CrossRef]
- Yuvaraj, K.; Bhattacharyya, M.; Prakash, R.; Ramkumar, V.; Ghosh, S. New Trinuclear Complexes of Group 6, 8, and 9 Metals with a Triply Bridging Borylene Ligand. Chem. Eur. J. 2016, 22, 8889–8896. [Google Scholar] [CrossRef]
- Bose, S.K.; Roy, D.K.; Shankhari, P.; Yuvaraj, K.; Mondal, B.; Sikder, A.; Ghosh, S. Syntheses and Characterization of New Vinyl-Borylene Complexes by the Hydroboration of Alkynes with [(μ3-BH)(Cp*RuCO)2(μ-CO)Fe(CO)3]. Chem. Eur. J. 2013, 19, 2337–2343. [Google Scholar] [CrossRef]
- Yuvaraj, K.; Roy, D.K.; Geetharani, K.; Mondal, B.; Anju, V.P.; Shankhari, P.; Ramkumar, V.; Ghosh, S. Chemistry of Homo- and Heterometallic Bridged-Borylene Complexes. Organometallics 2013, 32, 2705–2712. [Google Scholar] [CrossRef]
- Sharmila, D.; Mondal, B.; Ramalakshmi, R.; Kundu, S.; Varghese, B.; Ghosh, S. First-Row Transition-Metal–Diborane and –Borylene Complexes. Chem. Eur. J. 2015, 21, 5074–5083. [Google Scholar] [CrossRef]
- Saha, K.; Joseph, B.; Ramalakshmi, R.; Anju, R.S.; Varghese, B.; Ghosh, S. (η4-HBCC-σ,π-Borataallyl Complexes of Ruthenium Comprising an Agostic Interaction. Chem. Eur. J. 2016, 22, 7871–7878. [Google Scholar] [CrossRef]
- Bakthavachalam, K.; Yuvaraj, K.; Zafar, M.; Ghosh, S. Reactivity of [M2(μ-Cl)2(cod)2] (M=Ir, Rh) and [Ru(Cl)2(cod)(CH3CN)2] with Na[H2B(bt)2]: Formation of Agostic versus Borate Complexes. Chem. Eur. J. 2016, 22, 17291–17297. [Google Scholar] [CrossRef]
- Roy, D.K.; Mondal, B.; Anju, R.S.; Ghosh, S. Chemistry of Diruthenium and Dirhodium Analogues of Pentaborane(9): Synthesis and Characterization of Metal N,S-Heterocyclic Carbene and B-Agostic Complexes. Chem. Eur. J. 2015, 21, 3640–3648. [Google Scholar] [CrossRef]
- Saha, K.; Ramalakshmi, R.; Borthakur, R.; Gomosta, S.; Pathak, K.; Dorcet, V.; Roisnel, T.; Halet, J.-F.; Ghosh, S. An Efficient Method for the Synthesis of Boratrane Complexes of Late Transition Metals. Chem. Eur. J. 2017, 23, 18264–18275. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.K.; De, A.; Panda, S.; Varghese, B.; Ghosh, S. Chemistry of N,S-Heterocyclic Carbene and Metallaboratrane Complexes: A New η3-BCC-Borataallyl Complex. Chem. Eur. J. 2015, 21, 13732–13738. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Dhayal, R.S.; Varghese, B.; Ghosh, S. Unusual Open Eight-Vertex Oxamolybdaboranes: Structural Characterizations of (η5-C5Me5Mo)2B5(μ3-OEt) H6R (R = H and n-BuO). Organometallics 2009, 28, 1586–1589. [Google Scholar] [CrossRef]
- Sahoo, S.; Mobin, S.M.; Ghosh, S. Direct Insertion of Sulphur, Selenium and Tellurium atoms into Metallaborane Cages using Chalcogen Powders. J. Organomet. Chem. 2010, 695, 945–949. [Google Scholar] [CrossRef]
- Thakur, A.; Sao, S.; Ramkumar, V.; Ghosh, S. Novel Class of Heterometallic Cubane and Boride Clusters Containing Heavier Group 16 Elements. Inorg. Chem. 2012, 51, 8322–8330. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K. Reactivities of carbonyl sulfide (COS), carbon disulfide (CS2) and carbon dioxide (CO2) with transition metal complexes. Coord. Chem. Rev. 1995, 140, 37–114. [Google Scholar] [CrossRef]
- Busetto, L.; Palazzi, A.; Monari, M. Dithiocarbene complexes derived from CS2-bridged dinuclear complexes. J. Organomet. Chem. 1982, 228, C19–C20. [Google Scholar] [CrossRef]
- Ramalakshmi, R.; Roisnel, T.; Dorcet, V.; Halet, J.-F.; Ghosh, S. Synthesis and structural characterization of trithiocarbonate complexes of molybdenum and ruthenium derived from CS2 ligand. J. Organomet. Chem. 2017, 849–850, 256–260. [Google Scholar] [CrossRef]
- Mondal, B.; Bag, R.; Bakthavachalam, K.; Varghese, B.; Ghosh, S. Synthesis, Structures, and Characterization of Dimeric Neutral Dithiolato-Bridged Tungsten Complexes. Eur. J. Inorg. Chem. 2017, 5434–5441. [Google Scholar] [CrossRef]
- Rao, C.E.; Barik, S.K.; Yuvaraj, K.; Bakthavachalam, K.; Roisnel, T.; Dorcet, V.; Halet, J.-F.; Ghosh, S. Reactivity of CS2–Syntheses and Structures of Transition-Metal Species with Dithioformate and Methanedithiolate Ligands. Eur. J. Inorg. Chem. 2016, 4913–4920. [Google Scholar] [CrossRef]
- Anju, R.S.; Saha, K.; Mondal, B.; Roisnel, T.; Halet, J.-F.; Ghosh, S. In search for new bonding modes of the methylenedithiolato ligand: novel tri- and tetra-metallic clusters. Dalton Trans. 2015, 44, 11306–11313. [Google Scholar] [CrossRef]
- Dallanegra, R.; Chaplin, A.B.; Weller, A.S. Bis(σ-amine–borane) Complexes: An Unusual Binding Mode at a Transition-Metal Center. Angew. Chem. Int. Ed. 2009, 48, 6875–6878. [Google Scholar] [CrossRef]
- Marder, T.B.; Lin, Z. (Eds.) Contemporary Metal Boron Chemistry I: Borylenes, Boryls, Borane σ-Complexes, and Borohydrides; Springer-Verlag: Berlin, Germany, 2008; pp. 1–202. [Google Scholar]
- Kawano, Y.; Yamaguchi, K.; Miyake, S.; Kakizawa, T.; Shimoi, M. Investigation of the Stability of the M–H–B Bond in Borane σ Complexes [M(CO)5(η1-BH2R⋅L)] and [CpMn(CO)2(η1-BH2R⋅L)] (M = Cr, W; L = Tertiary Amine or Phosphine): Substituent and Lewis Base Effects. Chem. Eur. J. 2007, 13, 6920–6931. [Google Scholar] [CrossRef] [PubMed]
- Coffy, T.J.; Medford, G.; Plotkin, J.; Long, G.J.; Huffman, J.C.; Shore, S.G. Metalladiboranes of the iron subgroup: K[M(CO)4(η2-B2H5)] (μ-iron, ruthenium, osmium) and M′(η5-C5H5) (CO)2(η2-B2H5) (M′ = iron, ruthenium). Analogs of metal-olefin complexes). Organometallics 1989, 8, 2404–2409. [Google Scholar] [CrossRef]
- Plotkin, J.S.; Shore, S.G. Preparation of (η5-C5H5)(CO)2Fe(η2-B2H5): A neutral metallo-diborane(6) analogue of a metal–olefin complex. J. Organomet. Chem. 1979, 182, C15–C19. [Google Scholar] [CrossRef]
- Gloaguen, Y.; Alcaraz, G.; Pécharman, A.-F.; Clot, E.; Vendier, L.; Etienne, S.S. Phosphinoborane and Sulfidoborohydride as Chelating Ligands in Polyhydride Ruthenium Complexes: Agostic σ-Borane versus Dihydroborate Coordination. Angew. Chem. Int. Ed. 2009, 48, 2964–2968. [Google Scholar] [CrossRef]
- Hill, A.F.; Owen, G.R.; White, A.J.P.; Williams, D.J. The Sting of the Scorpion: A Metallaboratrane. Angew. Chem. Int. Ed. 1999, 38, 2759–2761. [Google Scholar] [CrossRef]
- Bontemps, S.; Gornitzka, H.; Bouhadir, G.; Miqueu, K.; Bourissou, D. Rhodium(I) Complexes of a PBP Ambiphilic Ligand: Evidence for a Metal→Borane Interaction. Angew. Chem. Int. Ed. 2006, 45, 1611–1614. [Google Scholar] [CrossRef]
- Figueroa, J.S.; Melnick, J.G.; Parkin, G. Reactivity of the Metal→BX3 Dative σ-Bond: 1,2-Addition Reactions of the Fe→BX3 Moiety of the Ferraboratrane Complex [κ4-B(mimBut)3]Fe(CO)2. Inorg. Chem. 2006, 45, 7056–7058. [Google Scholar] [CrossRef] [PubMed]
- Westcott, S.A.; Marder, T.B.; Baker, R.T.; Harlow, R.L.; Calabrese, J.C.; Lam, K.C.; Lin, Z. Reactions of hydroborating reagents with phosphinorhodium hydride complexes: molecular structures of a Rh2B3 metallaborane cluster, an L2Rh(η2-H2BR2) complex and a mixed valence Rh dimer containing a semi-bridging Bcat (cat = 1,2-O2C6H4) group. Polyhedron 2004, 23, 2665–2677. [Google Scholar] [CrossRef]
- Braunschweig, H.; Radacki, K.; Rais, D.; Whittell, G.R. A Boryl Bridged Complex: An Unusual Coordination Mode of the BR2 Ligand. Angew. Chem. Int. Ed. 2005, 44, 1192–1194. [Google Scholar] [CrossRef]
- Feilong, J.; Fehlner, T.P.; Rheingold, A.L. Preparation of 2,3,4-Tris(η5-cyclopentadienyl)-1,5-diphenyl-1-phospha-2,3,4-tricobaltapentaborane(5); Phenyl Group Migration from Phosphorus to Boron. Angew. Chem. Int. Ed. Engl. 1988, 27, 424–426. [Google Scholar] [CrossRef]
- Ibers, J.A. Centenary Lecture. Reactivities of carbon disulphide, carbon dioxide, and carbonyl sulphide towards some transition-metal systems. Chem. Soc. Rev. 1982, 11, 57–73. [Google Scholar] [CrossRef]
- Choy, V.J.; O’Connor, C.J. Chelating dioxygen compounds of the platinum metals. Coord. Chem. Rev. 1972, 9, 145–170. [Google Scholar] [CrossRef]
- Walther, D. Homogeneous-catalytic reactions of carbon dioxide with unsatureated substrates, reversible CO2-carriers and transcarboxylation reactions. Coord. Chem. Rev. 1987, 79, 135–174. [Google Scholar] [CrossRef]
- Anju, R.S.; Saha, K.; Mondal, B.; Dorcet, V.; Roisnel, T.; Halet, J.-F.; Ghosh, S. Chemistry of Diruthenium Analogue of Pentaborane(9) With Heterocumulenes: Toward Novel Trimetallic Cubane-Type Clusters. Inorg. Chem. 2014, 53, 10527–10535. [Google Scholar] [CrossRef] [PubMed]
- Coldicott, R.S.; Kennedy, J.D.; Pett, M.T.J. Reactions of carbon disulfide with open nido-6-iridadecaboranes. The formation of closed ten-vertex cluster compounds with boron-to-metal dithioformate bridges and a novel isocloso→closo cluster conversion. J. Chem. Soc. Dalton Trans. 1996, 3819–3824. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shang, M.; Fehlner, T.P. Reactions of an Electronically Unsaturated Chromaborane. Coordination of CS2 to (η5-C5Me5)2Cr2B4H8 and Its Hydroboration to a Methanedithiolato Ligand. Organometallics 1996, 15, 1963–1965. [Google Scholar] [CrossRef]
- Hartwig, J.F.; Huber, S. Transition metal boryl complexes: structure and reactivity of CpFe(CO)2Bcat and CpFe(CO)2BPh2. J. Am. Chem. Soc. 1993, 115, 4908–4909. [Google Scholar] [CrossRef]
- Westcott, A.S.; Marder, T.B.; Baker, R.T. Transition metal-catalyzed addition of catecholborane to α-substituted vinylarenes: hydroboration vs. dehydrogenative borylation. Organometallics 1993, 12, 975–979. [Google Scholar] [CrossRef]
- Evans, D.A.; Fu, G.C.; Hoveyda, A.H. Rhodium(I)- and iridium(I)-catalyzed hydroboration reactions: scope and synthetic applications. J. Am. Chem. Soc. 1992, 114, 6671–6679. [Google Scholar] [CrossRef]
- Auerhammer, D.; Arrowsmith, M.; Dewhurst, R.D.; Kupfer, T.; Böhnke, J.; Braunschweig, H. Closely related yet different: A borylene and its dimer are non-interconvertible but connected through reactivity. Chem. Sci. 2018, 9, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Habben, C.; Meller, A.; Noltemeyer, M.; Sheldrick, G.M. Synthese, Molekül- und Kristallstruktur von 3,5-Dimethyl-2,6-bistrimethylsilyl-l-thia-2,4,6-triaza-3,5-diborinan-wolframpentacarbonyl. Z. Naturforsch. 1986, 41b, 799–802. [Google Scholar] [CrossRef]
- Matsubara, H.; Tanaka, T.; Takai, Y.; Sawada, M.; Seto, K.; Imazaki, H.; Takahashi, S. Structural Studies of a Liquid Crystalline Compound, 2-(4-Cyanophenyl)-5-(4-butylphenyl)-1,3,2-dioxaborinane, by Means of Nuclear Magnetic Resonance and X-Ray Analyses. Bull. Chem. Soc. Jpn. 1991, 64, 2103–2108. [Google Scholar] [CrossRef] [Green Version]
- Slabber, C.A.; Grimmer, C.; Akerman, M.P.; Robinson, R.S. 2-Phenylnaphtho[1,8-de][1,3,2]diazaborinane. Acta Cryst. 2011, E67, o1995. [Google Scholar] [CrossRef] [PubMed]
- Wade, K. Structural and Bonding Patterns in Cluster Chemistry. Adv. Inorg. Chem. Radiochem. 1976, 18, 1–66. [Google Scholar] [CrossRef]
- Mingos, D.M.P. A General Theory for Cluster and Ring Compounds of the Main Group and Transition Elements. Nat. Phys. Sci. 1972, 236, 99–102. [Google Scholar] [CrossRef]
- Mingos, D.M.P. Polyhedral skeletal electron pair approach. Acc. Chem. Res. 1984, 17, 311–319. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Balakrishnarajan, M.N.; Pancharatna, P.D. Electronic Requirements for Macropolyhedral Boranes. Chem. Rev. 2002, 102, 93–144. [Google Scholar] [CrossRef]
- Ryschkewitsch, G.E.; Nainan, K.C. Octahydrotriborate (1-) ([B3H8]) salts. Inorg. Synth. 1974, 15, 113–114. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Altornare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Cryst. 1993, 26, 343–350. [Google Scholar] [CrossRef]





| Entry | 11B NMR (ppm) a | dav[B–E] b [Å] | Conformations c |
|---|---|---|---|
 | 8.3 d | 1.352 | half chair |
 | −5.0 and −15.6 | 1.915 | chair |
 | f | 1.414 | planar |
 | 37.6 | 1.433 | boat |
 | −11.2 e | 1.943 | boat |
 | −4.1 | 1.919 | boat |
 | 7.3 (3) 4.9 (4) | 1.923 f | Boat f |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, K.; Kaur, U.; Borthakur, R.; Ghosh, S. Synthesis of Trithia-Borinane Complexes Stabilized in Diruthenium Core: [(Cp*Ru)2(η1-S)(η1-CS){(CH2)2S3BR}] (R = H or SMe). Inorganics 2019, 7, 21. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020021
Saha K, Kaur U, Borthakur R, Ghosh S. Synthesis of Trithia-Borinane Complexes Stabilized in Diruthenium Core: [(Cp*Ru)2(η1-S)(η1-CS){(CH2)2S3BR}] (R = H or SMe). Inorganics. 2019; 7(2):21. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020021
Chicago/Turabian StyleSaha, Koushik, Urminder Kaur, Rosmita Borthakur, and Sundargopal Ghosh. 2019. "Synthesis of Trithia-Borinane Complexes Stabilized in Diruthenium Core: [(Cp*Ru)2(η1-S)(η1-CS){(CH2)2S3BR}] (R = H or SMe)" Inorganics 7, no. 2: 21. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020021