Chiral Self-Sorting in Truxene-Based Metallacages
Abstract
:1. Introduction
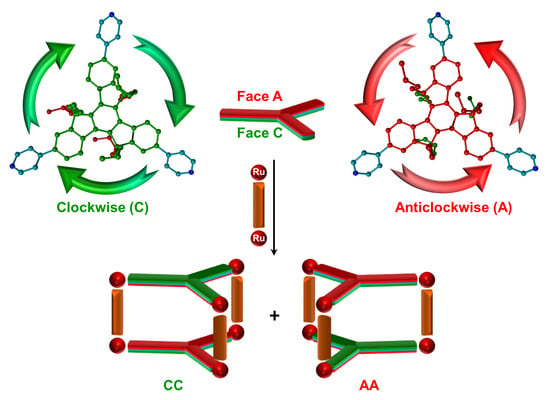
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Experimental Procedure and Characterization Data
3.4. X-Ray Crystallographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, B.; He, T.; Fan, Y.; Yuan, X.; Qiu, H.; Yin, S. Recent developments in the construction of metallacycle/metallacage-cored supramolecular polymers via hierarchical self-assembly. Chem. Commun. 2019, 55, 8036–8059. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.-M.; Liu, J.-Q.; Zhang, L.; Wang, Y.-Y.; Hahn, F.E.; Han, Y.-F. Preparation and Post-Assembly Modification of Metallosupramolecular Assemblies from Poly(N-Heterocyclic Carbene) Ligands. Chem. Rev. 2018, 118, 9587–9641. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, H.-N.; Jin, G.-X. Molecular Borromean Rings Based on Half-Sandwich Organometallic Rectangles. Acc. Chem. Res. 2018, 51, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Pilgrim, B.S.; Nitschke, J.R. Covalent post-assembly modification in metallosupramolecular chemistry. Chem. Soc. Rev. 2018, 47, 626–644. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.-Y.; Chen, L.-J.; Xu, L.; Zhao, X.-L.; Yang, H.-B. Construction of supramolecular hexagonal metallacycles via coordination-driven self-assembly: Structure, properties and application. Coord. Chem. Rev. 2018, 369, 39–75. [Google Scholar] [CrossRef]
- Huang, S.-L.; Hor, T.S.A.; Jin, G.-X. Metallacyclic assembly of interlocked superstructures. Coord. Chem. Rev. 2017, 333, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.; Johnstone, M.D.; Clever, G.H. Interpenetrated Cage Structures. Chem. Eur. J. 2016, 22, 14104–14125. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Henkelis, J.J.; Hardie, M.J. Controlling the assembly of cyclotriveratrylene-derived coordination cages. Chem. Commun. 2015, 51, 11929–11943. [Google Scholar] [CrossRef] [Green Version]
- Bilbeisi, R.A.; Olsen, J.-C.; Charbonnière, L.J.; Trabolsi, A. Self-assembled discrete metal–organic complexes: Recent advances. Inorg. Chim. Acta 2014, 417, 79–108. [Google Scholar] [CrossRef]
- Mishra, A.; Gupta, R. Supramolecular architectures with pyridine-amide based ligands: Discrete molecular assemblies and their applications. Dalton Trans. 2014, 43, 7668–7682. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukherjee, P.S. Template-free multicomponent coordination-driven self-assembly of Pd(II)/Pt(II) molecular cages. Chem. Commun. 2014, 50, 2239–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanasekaran, P.; Lee, C.-H.; Lu, K.-L. Neutral discrete metal–organic cyclic architectures: Opportunities for structural features and properties in confined spaces. Coord. Chem. Rev. 2014, 280, 96–175. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Klosterman, J.K. Molecular architectures of multi-anthracene assemblies. Chem. Soc. Rev. 2014, 43, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kang, S.C.; Chi, K.-W. Coordination-Driven Self-Assembly of Arene–Ruthenium Compounds. Eur. J. Inorg. Chem. 2013, 2013, 5222–5232. [Google Scholar] [CrossRef]
- Ward, M.D.; Raithby, P.R. Functional behaviour from controlled self-assembly: Challenges and prospects. Chem. Soc. Rev. 2013, 42, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-X.; Zhou, Q.-F.; Jiang, S.-T.; Zhang, Y.; Yin, G.-Q.; Jiang, B.; Li, X.; Tan, H.; Yang, H.-B. Photoresponsive Chirality-Tunable Supramolecular Metallacycles. Macromol. Rapid Commun. 2018, 39, 1800454. [Google Scholar] [CrossRef]
- Diaz-Moscoso, A.; Ballester, P. Light-responsive molecular containers. Chem. Commun. 2017, 53, 4635–4652. [Google Scholar] [CrossRef]
- Saha, M.L.; Yan, X.; Stang, P.J. Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and its Applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive Host–Guest Functional Systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef] [PubMed]
- Zarra, S.; Wood, D.M.; Roberts, D.A.; Nitschke, J.R. Molecular containers in complex chemical systems. Chem. Soc. Rev. 2015, 44, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krykun, S.; Dekhtiarenko, M.; Canevet, D.; Carre, V.; Aubriet, F.; Levillain, E.; Allain, M.; Voitenko, Z.; Sallé, M.; Goeb, S. Metalla-Assembled Electron-Rich Tweezers: Redox-Controlled Guest Release Through Supramolecular Dimerization. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef] [PubMed]
- Plessius, R.; Orth, N.; Ivanović-Burmazović, I.; Siegler, M.A.; Reek, J.N.H.; van der Vlugt, J.I. Reversible multi-electron storage in dual-site redox-active supramolecular cages. Chem. Commun. 2019, 55, 12619–12622. [Google Scholar] [CrossRef] [PubMed]
- Szalóki, G.; Krykun, S.; Croué, V.; Allain, M.; Morille, Y.; Aubriet, F.; Carré, V.; Voitenko, Z.; Goeb, S.; Sallé, M. Redox-Driven Transformation of a Discrete Molecular Cage into an Infinite 3D Coordination Polymer. Chem. Eur. J. 2018, 24, 11273–11277. [Google Scholar] [CrossRef]
- Szalóki, G.; Croué, V.; Carré, V.; Aubriet, F.; Alévêque, O.; Levillain, E.; Allain, M.; Arago, J.; Orti, E.; Goeb, S.; et al. Controlling the Host-Guest Interaction Mode through a Redox Stimulus. Angew. Chem. Int. Ed. 2017, 56, 16272–16276. [Google Scholar] [CrossRef] [Green Version]
- Croué, V.; Goeb, S.; Szalóki, G.; Allain, M.; Sallé, M. Reversible Guest Uptake/Release by Redox-Controlled Assembly/Disassembly of a Coordination Cage. Angew. Chem. Int. Ed. 2016, 55, 1746–1750. [Google Scholar] [CrossRef] [Green Version]
- Croué, V.; Goeb, S.; Sallé, M. Metal-driven self-assembly: The case of redox-active discrete architectures. Chem. Commun. 2015, 51, 7275–7289. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.X.; Chen, L.J.; Yang, H.B. Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: From structure to functions. Chem. Soc. Rev. 2015, 44, 2148–2167. [Google Scholar] [CrossRef]
- Bivaud, S.; Goeb, S.; Croué, V.; Dron, P.I.; Allain, M.; Sallé, M. Self-Assembled Containers Based on Extended Tetrathiafulvalene. J. Am. Chem. Soc. 2013, 135, 10018–10021. [Google Scholar] [CrossRef]
- Bivaud, S.; Balandier, J.Y.; Chas, M.; Allain, M.; Goeb, S.; Sallé, M. A Metal-Directed Self-Assembled Electroactive Cage with Bis(pyrrolo)tetrathiafulvalene (BPTTF) Side Walls. J. Am. Chem. Soc. 2012, 134, 11968–11970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Chen, Q.; Wu, M.; Jiang, F.; Hong, M. Controllable Coordination-Driven Self-Assembly: From Discrete Metallocages to Infinite Cage-Based Frameworks. Acc. Chem. Res. 2015, 48, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Yang, H.-B. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Vasdev, R.A.S.; Preston, D.; Crowley, J.D. Strategies for Reversible Guest Uptake and Release from Metallosupramolecular Architectures. Chem. Eur. J. 2018, 24, 14878–14890. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal-organic molecular cages: Applications of biochemical implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-Responsive Metal–Ligand Assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.-X.; Yang, H.-B. Recent advances in the construction of fluorescent metallocycles and metallocages via coordination-driven self-assembly. Dalton Trans. 2015, 44, 867–890. [Google Scholar] [CrossRef]
- Therrien, B. Discrete Metalla-Assemblies as Drug Delivery Vectors. In Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering; John Wiley & Sons, Inc.: New York, NY, USA, 2013; pp. 145–166. [Google Scholar] [CrossRef]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined Nanospaces in Metallocages: Guest Molecules, Weakly Encapsulated Anions, and Catalyst Sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef]
- Therrien, B. Drug Delivery by Water-Soluble Organometallic Cages. In Chemistry of Nanocontainers; Albrecht, M., Hahn, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 319, pp. 35–55. [Google Scholar]
- Chen, L.-J.; Zhu, J.-L.; Yang, H.-B. CHAPTER 4 Self-assembled Chiral Metallomacrocycles. In Metallomacrocycles: from Structures to Applications; The Royal Society of Chemistry: London, UK, 2019; pp. 77–105. [Google Scholar] [CrossRef]
- Chen, L.-J.; Yang, H.-B.; Shionoya, M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 2017, 46, 2555–2576. [Google Scholar] [CrossRef]
- Jędrzejewska, H.; Szumna, A. Making a Right or Left Choice: Chiral Self-Sorting as a Tool for the Formation of Discrete Complex Structures. Chem. Rev. 2017, 117, 4863–4899. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Castilla, A.M.; Ramsay, W.J.; Nitschke, J.R. Stereochemistry in Subcomponent Self-Assembly. Acc. Chem. Res. 2014, 47, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.M.; Clever, G.H. Integrative self-sorting of coordination cages based on ‘naked’ metal ions. Chem. Commun. 2017, 53, 8506–8516. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Jiang, W.; Schalley, C.A. Integrative self-sorting: A versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 2015, 44, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Safont-Sempere, M.M.; Fernández, G.; Würthner, F. Self-Sorting Phenomena in Complex Supramolecular Systems. Chem. Rev. 2011, 111, 5784–5814. [Google Scholar] [CrossRef]
- Schulte, T.R.; Holstein, J.J.; Clever, G.H. Chiral Self-Discrimination and Guest Recognition in Helicene-Based Coordination Cages. Angew. Chem. Int. Ed. 2019, 58, 5562–5566. [Google Scholar] [CrossRef]
- Beaudoin, D.; Rominger, F.; Mastalerz, M. Chiral Self-Sorting of [2 + 3] Salicylimine Cage Compounds. Angew. Chem. Int. Ed. 2017, 56, 1244–1248. [Google Scholar] [CrossRef]
- Rota Martir, D.; Escudero, D.; Jacquemin, D.; Cordes, D.B.; Slawin, A.M.Z.; Fruchtl, H.A.; Warriner, S.L.; Zysman-Colman, E. Homochiral Emissive Λ8- and Δ8-[Ir8Pd4]16+ Supramolecular Cages. Chem. Eur. J. 2017, 23, 14358–14366. [Google Scholar] [CrossRef] [Green Version]
- Boer, S.A.; Turner, D.R. Self-selecting homochiral quadruple-stranded helicates and control of supramolecular chirality. Chem. Commun. 2015, 51, 17375–17378. [Google Scholar] [CrossRef]
- Maeda, C.; Kamada, T.; Aratani, N.; Osuka, A. Chiral self-discriminative self-assembling of meso–meso linked diporphyrins. Coord. Chem. Rev. 2007, 251, 2743–2752. [Google Scholar] [CrossRef]
- Lützen, A.; Hapke, M.; Griep-Raming, J.; Haase, D.; Saak, W. Synthesis and Stereoselective Self-Assembly of Double- and Triple-Stranded Helicates. Angew. Chem. Int. Ed. 2002, 41, 2086–2089. [Google Scholar] [CrossRef]
- Masood, M.A.; Enemark, E.J.; Stack, T.D.P. Ligand Self-Recognition in the Self-Assembly of a [{Cu(L)}2]2+ Complex: The Role of Chirality. Angew. Chem. Int. Ed. 1998, 37, 928–932. [Google Scholar] [CrossRef]
- Arribas, C.S.; Wendt, O.F.; Sundin, A.P.; Carling, C.-J.; Wang, R.; Lemieux, R.P.; Wärnmark, K. Formation of an heterochiral supramolecular cage by diastereomer self-discrimination: Fluorescence enhancement and C60 sensing. Chem. Commun. 2010, 46, 4381–4383. [Google Scholar] [CrossRef] [Green Version]
- Weilandt, T.; Kiehne, U.; Schnakenburg, G.; Lützen, A. Diastereoselective self-assembly of dinuclear heterochiral metallosupramolecular rhombs in a self-discriminating process. Chem. Commun. 2009, 17, 2320–2322. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Greenfield, J.L.; Brotin, T.; Berthault, P.; Léonce, E.; Zhu, J.-L.; Xu, L.; Nitschke, J.R. Enantiopure [Cs+/Xe⊂Cryptophane]⊂FeII4L4 Hierarchical Superstructures. J. Am. Chem. Soc. 2019, 141, 8339–8345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, X.; Xuan, W.; Peng, P.; Li, Z.; Lu, R.; Wu, S.; Tian, Z.; Cao, X. Chiral separation and characterization of triazatruxene-based face-rotating polyhedra: The role of non-covalent facial interactions. Chem. Commun. 2018, 54, 4685–4688. [Google Scholar] [CrossRef]
- Qu, H.; Tang, X.; Wang, X.; Li, Z.; Huang, Z.; Zhang, H.; Tian, Z.; Cao, X. Chiral molecular face-rotating sandwich structures constructed through restricting the phenyl flipping of tetraphenylethylene. Chem. Sci. 2018, 9, 8814–8818. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, H.; Tranca, I.; Qu, H.; Wang, X.; Markvoort, A.J.; Tian, Z.; Cao, X. Elucidation of the origin of chiral amplification in discrete molecular polyhedra. Nat. Commun. 2018, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Peng, P.; Xuan, W.; Wang, Y.; Zhuang, Y.; Tian, Z.; Cao, X. Narcissistic chiral self-sorting of molecular face-rotating polyhedra. Org. Biomol. Chem. 2018, 16, 34–37. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, H.; Zhang, W.; Zhuang, Y.; Tian, Z.; Cao, X. Interconversion of molecular face-rotating polyhedra through turning inside out. Chem. Commun. 2017, 53, 8956–8959. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Yang, H.; Fang, H.; Chen, R.; Sun, Y.; Zheng, N.; Tan, K.; Lu, X.; Tian, Z.; et al. Assembled molecular face-rotating polyhedra to transfer chirality from two to three dimensions. Nat. Commun. 2016, 7, 12469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bols, P.S.; Anderson, H.L. Shadow Mask Templates for Site-Selective Metal Exchange in Magnesium Porphyrin Nanorings. Angew. Chem. Int. Ed. 2018, 57, 7874–7877. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Lalevée, J.; Telitel, S.; Contal, E.; Dumur, F.; Gigmes, D.; Bertin, D.; Nechab, M.; Graff, B.; Morlet-Savary, F.; et al. Polyaromatic Structures as Organo-Photoinitiator Catalysts for Efficient Visible Light Induced Dual Radical/Cationic Photopolymerization and Interpenetrated Polymer Networks Synthesis. Macromolecules 2012, 45, 4454–4460. [Google Scholar] [CrossRef]
- Kanibolotsky, A.L.; Berridge, R.; Skabara, P.J.; Perepichka, I.F.; Bradley, D.D.C.; Koeberg, M. Synthesis and Properties of Monodisperse Oligofluorene-Functionalized Truxenes: Highly Fluorescent Star-Shaped Architectures. J. Am. Chem. Soc. 2004, 126, 13695–13702. [Google Scholar] [CrossRef]
- Guan, J.; Xu, F.; Tian, C.; Pu, L.; Yuan, M.-S.; Wang, J. Tricolor Luminescence Switching by Thermal and Mechanical Stimuli in the Crystal Polymorphs of Pyridyl-substituted Fluorene. Chem. Asian J. 2019, 14, 216–222. [Google Scholar] [CrossRef]
- Therrien, B. Arene Ruthenium Cages: Boxes Full of Surprises. Eur. J. Inorg. Chem. 2009, 17, 2445–2453. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Avram, L.; Frish, L. Diffusion NMR Spectroscopy in Supramolecular and Combinatorial Chemistry: An Old Parameter-New Insights. Angew. Chem. Int. Ed. 2005, 44, 520–554. [Google Scholar] [CrossRef]
- Kim, T.; Singh, N.; Oh, J.; Kim, E.-H.; Jung, J.; Kim, H.; Chi, K.-W. Selective Synthesis of Molecular Borromean Rings: Engineering of Supramolecular Topology via Coordination-Driven Self-Assembly. J. Am. Chem. Soc. 2016, 138, 8368–8371. [Google Scholar] [CrossRef]
- Fazio, E.; Haynes, C.J.E.; de la Torre, G.; Nitschke, J.R.; Torres, T. A giant M2L3 metallo-organic helicate based on phthalocyanines as a host for electroactive molecules. Chem. Commun. 2018, 54, 2651–2654. [Google Scholar] [CrossRef] [Green Version]
- García-Frutos, E.M.; Gómez-Lor, B.; Monge, Á.; Gutiérrez-Puebla, E.; Alkorta, I.; Elguero, J. Synthesis and Preferred All-syn Conformation of C3-Symmetrical N-(Hetero)arylmethyl Triindoles. Chem. Eur. J. 2008, 14, 8555–8561. [Google Scholar] [CrossRef] [PubMed]
- Mirtschin, S.; Slabon-Turski, A.; Scopelliti, R.; Velders, A.H.; Severin, K. A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc. 2010, 132, 14004–14005. [Google Scholar] [CrossRef] [PubMed]
- Govindaswamy, P.; Linder, D.; Lacour, J.; Süss-Fink, G.; Therrien, B. Self-assembled hexanuclear arene ruthenium metallo-prisms with unexpected double helical chirality. Chem. Commun. 2006, 45, 4691–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailar, J.C. Some problems in the stereochemistry of coordination compounds: Introductory lecture. J. Inorg. Nucl. Chem. 1958, 8, 165–175. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Furrer, J.; Therrien, B. In- and Out-of-Cavity Interactions by Modulating the Size of Ruthenium Metallarectangles. Helv. Chim. Acta 2010, 93, 1313–1328. [Google Scholar] [CrossRef] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Séjourné, S.; Labrunie, A.; Dalinot, C.; Benchohra, A.; Carré, V.; Aubriet, F.; Allain, M.; Sallé, M.; Goeb, S. Chiral Self-Sorting in Truxene-Based Metallacages. Inorganics 2020, 8, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8010001
Séjourné S, Labrunie A, Dalinot C, Benchohra A, Carré V, Aubriet F, Allain M, Sallé M, Goeb S. Chiral Self-Sorting in Truxene-Based Metallacages. Inorganics. 2020; 8(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8010001
Chicago/Turabian StyleSéjourné, Simon, Antoine Labrunie, Clément Dalinot, Amina Benchohra, Vincent Carré, Frédéric Aubriet, Magali Allain, Marc Sallé, and Sébastien Goeb. 2020. "Chiral Self-Sorting in Truxene-Based Metallacages" Inorganics 8, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8010001