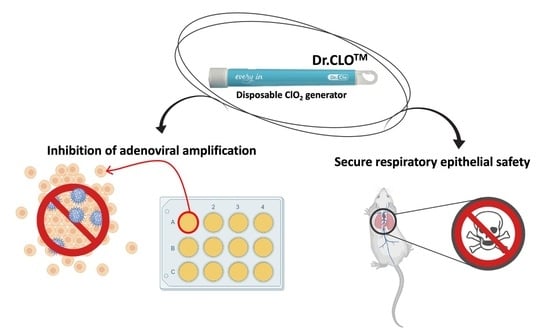
Respiratory Safety Evaluation in Mice and Inhibition of Adenoviral Amplification in Human Bronchial Endothelial Cells Using a Novel Type of Chlorine Dioxide Gas Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concentration and Duration of Chlorine Dioxide (ClO2) Gas Generation by Dr.CLOTM
2.2. Measurement of Dissolved Cl− Ions in Culture Medium
2.3. Adenoviral Infectivity Titer
2.3.1. Cell Culture
2.3.2. Viral Forming Units (Titer)
2.3.3. Inhibition of Functional Titer Virus Infectivity by ELISA
2.4. Animal Preparation
2.5. Study Designs for Single ClO2 Gas Whole-Body Exposure in Chambers
- Material: acryl;
- Floor: SUS304 stainless steel;
- Air in & out ventilation count: 12 times/h.
2.5.1. Maintaining ClO2 Gas Environment during the Study
2.5.2. Groups for Single ClO2 Gas Exposure in Chambers
2.6. Histopathologic Evaluation and Scoring of Lung Alveolar Epithelium
2.7. Statistics
3. Results
3.1. ClO2 Gas Concentrations and Duration by Dr.CLOTM
3.2. Dissolved Chloride Concentration in Culture Medium
3.3. Calculation of Adenoviral Infection Titer (Infectious Unitw/mL) in Human Respiratory Epithelial Cells
3.4. Inhibition of Adenoviral Infection (ELISA Method)
3.5. Animal Body Weight Changes
3.6. Animal Death after High Concentrations of Single ClO2 Gas Inhalation to Determine LC50
3.7. Histopathological Findings
3.7.1. H&E Staining and Masson’s Trichrome Staining
3.7.2. Histopathological Examination Scoring for Lung
3.7.3. Area Comparison of the Alveolar Air Sac
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Biswal, M.; Kanaujia, R.; Angrup, A.; Ray, P.; Singh, S.M. Disinfection tunnels: Potentially counterproductive in the context of a prolonged pandemic of COVID-19. Public Health 2020, 183, 48. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Schnall, A.H.; Law, R.; Bronstein, A.C.; Marraffa, J.M.; Spiller, H.A.; Hays, H.L.; Funk, A.R.; Mercurio-Zappala, M.; Calello, D.P. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19—National poison data system, United States, 1 January 2020–31 March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 496. [Google Scholar] [CrossRef] [Green Version]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and effective use of ozone as air and surface disinfectant in the conjuncture of COVID-19. Gases 2021, 1, 19–32. [Google Scholar] [CrossRef]
- Guridi, A.; Sevillano, E.; De La Fuente, I.; Mateo, E.; Eraso, E.; Quindós, G. Disinfectant activity of a portable ultraviolet C equipment. Int. J. Environ. Res. Public Health 2019, 16, 4747. [Google Scholar] [CrossRef] [Green Version]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: www.cdc.gov/infectioncontrol/guidelines/disinfection/ (accessed on 31 October 2021).
- Rutala, W.A.; Weber, D.J. Sterilization, high-level disinfection, and environmental cleaning. Infect. Dis. Clin. 2011, 25, 45–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Chen, Y.-H.; Zhao, Y.-L.; Chiang, P.-C. Application of Gaseous ClO2 on Disinfection and Air Pollution Control: A Mini Review. Aerosol Air Qual. Res. 2020, 20, 2289–2298. [Google Scholar] [CrossRef]
- Kály-Kullai, K.; Wittmann, M.; Noszticzius, Z.; Rosivall, L. Can chlorine dioxide prevent the spreading of coronavirus or other viral infections? Medical hypotheses. Physiol. Int. 2020, 107, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liester, M.B. The chlorine dioxide controversy: A deadly poison or a cure for COVID-19? Int. J. Med. Med. Sci. 2021, 13, 13–21. [Google Scholar]
- Rai, N.K.; Ashok, A.; Akondi, B.R. Consequences of chemical impact of disinfectants: Safe preventive measures against COVID-19. Crit. Rev. Toxicol. 2020, 50, 513–520. [Google Scholar] [CrossRef]
- Al-Sa’ady, A.T.; Nahar, H.S.; Saffah, F.F. Antibacterial activities of chlorine gas and chlorine dioxide gas against some pathogenic bacteria. EurAsian Journal of Biosciences 2020, 14, 3875–3882. [Google Scholar]
- Young, R.O. Chlorine dioxide (ClO2) as a non-toxic antimicrobial agent for virus, bacteria and yeast (Candida albicans). Int. J. Vaccines Vaccin. 2016, 2, 00052. [Google Scholar] [CrossRef] [Green Version]
- Girouard Jr, D.J.; Czarneski, M.A. Room, suite scale, class III biological safety cabinet, and sensitive equipment decontamination and validation using gaseous chlorine dioxide. Appl. Biosaf. 2016, 21, 34–44. [Google Scholar] [CrossRef]
- Wood, J.P.; Ryan, S.P.; Snyder, E.G.; Serre, S.D.; Touati, A.; Clayton, M.J. Adsorption of chlorine dioxide gas on activated carbons. J. Air Waste Manag. Assoc. 2010, 60, 898–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, C.N. Decontamination using chlorine dioxide. In Hearings on the Decontamination of Anthrax and other Biological Agents; Committee on Science, United States House of Representatives: Washington, DC, USA, 2001; pp. 2001–2002. [Google Scholar]
- Akamatsu, A.; Lee, C.; Morino, H.; Miura, T.; Ogata, N.; Shibata, T. Six-month low level chlorine dioxide gas inhalation toxicity study with two-week recovery period in rats. J. Occup. Med. Toxicol. 2012, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, N.; Shibata, T. Protective effect of low-concentration chlorine dioxide gas against influenza A virus infection. J. Gen. Virol. 2008, 89 Pt 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Sakasegawa, M.; Miura, T.; Shibata, T.; Takigawa, Y.; Taura, K.; Taguchi, K.; Matsubara, K.; Nakahara, K.; Kato, D. Inactivation of airborne bacteria and viruses using extremely low concentrations of chlorine dioxide gas. Pharmacology 2016, 97, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Mimura, S.; Fujioka, T.; Mitsumaru, A. Preventive effect against influenza-like illness by low-concentration chlorine dioxide gas. Jpn. J. Environ. Infect. 2010, 25, 277–280. [Google Scholar] [CrossRef]
- Das, R.; Blanc, P.D. Chlorine gas exposure and the lung: A review. Toxicol. Ind. Health 1993, 9, 439–455. [Google Scholar] [CrossRef]
- Gunnarsson, M.; Walther, S.M.; Seidal, T.; Bloom, G.D.; Lennquist, S. Exposure to chlorine gas: Effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J. Appl. Toxicol. 1998, 18, 249–255. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Kuschner, W.; Wong, H.; Boushey, H.A.; Blanc, P.D. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 1996, 109, 331–337. [Google Scholar] [CrossRef]
- Hatanaka, N.; Xu, B.; Yasugi, M.; Morino, H.; Tagishi, H.; Miura, T.; Shibata, T.; Yamasaki, S. Chlorine dioxide is a more potent antiviral agent against SARS-CoV-2 than sodium hypochlorite. J. Hosp. Infect. 2021, 118, 20–26. [Google Scholar] [CrossRef]
- Burela, A.; Hernández-Vásquez, A.; Comandé, D.; Peralta, V.; Fiestas, F. Chlorine dioxide and chlorine derivatives for the prevention or treatment of COVID-19: A systematic review. Rev. Peru. Med. Exp. Salud Publica 2021, 37, 605–610. [Google Scholar] [CrossRef]
- White, C.W.; Martin, J.G. Chlorine gas inhalation: Human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc. 2010, 7, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sangion, A.; Li, L. Evaluating consumer exposure to disinfecting chemicals against coronavirus disease 2019 (COVID-19) and associated health risks. Environ. Int. 2020, 145, 106108. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of emerging contaminants and associated human health effects. Biomed. Res. Int. 2015, 2015, 404796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, N.; Koizumi, T.; Ozawa, F. Ten-week Whole-body Inhalation Toxicity Study of Chlorine Dioxide Gas in Rats. J. Drug Metab. Toxicol. 2013, 4, 143. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Kim, T.; Yoon, H.; Jo, A.; Lee, D.; Kim, P.; Seo, J. Health risk assessment of dermal and inhalation exposure to deodorants in Korea. Sci. Total Environ. 2018, 625, 1369–1379. [Google Scholar] [CrossRef]
- Han, D.-H.; Chung, S.-T.; Kim, J.-i.; Cho, Y.-S.; Lee, C.-S. A Study on Selecting Personal Protective Equipment for Listed Hazardous Chemicals (2): Analysis Using an Exposure Risk Matrix. J. Environ. Health Sci. 2016, 42, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Food and Drug Safety, Korea (Ed.) Standard for Toxicity Study of Pharmaceuticals. In MFDS Notification No. 2015, 82th ed.; National Institute of Food and Drug Safety Evaluation: Cheongju, Korea, 2015; p. 50. [Google Scholar]
- Nakashima, H.; Omae, K.; Takebayashi, T.; Ishizuka, C.; Sakurai, H.; Yamazaki, K.; Nakaza, M.; Shibata, T.; Kudo, M.; Koshi, S. Acute and subacute inhalation toxicity of dichlorosilane in male ICR mice. Arch. Toxicol. 1996, 70, 218–223. [Google Scholar] [CrossRef]
- Uemura, T.; Omae, K.; Nakashima, H.; Sakurai, H.; Yamazaki, K.; Shibata, T.; Mori, K.; Kudo, M.; Kanoh, H.; Tati, M. Acute and subacute inhalation toxicity of diborane in male ICR mice. Arch. Toxicol. 1995, 69, 397–404. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Committee on Acute Exposure Guideline Levels. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 9; National Academies Press: Washington, DC, USA, 2009.
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 3; National Academies Press: Washington, DC, USA, 2003.
- Calikoglu, M.; Tamer, L.; Sucu, N.; Coskun, B.; Ercan, B.; Gul, A.; Calikoglu, I.; Kanik, A. The effects of caffeic acid phenethyl ester on tissue damage in lung after hindlimb ischemia-reperfusion. Pharmacol. Res. 2003, 48, 397–403. [Google Scholar] [CrossRef]
- Turut, H.; Ciralik, H.; Kilinc, M.; Ozbag, D.; Imrek, S.S. Effects of early administration of dexamethasone, N-acetylcysteine and aprotinin on inflammatory and oxidant-antioxidant status after lung contusion in rats. Injury 2009, 40, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Basaran, U.N.; Ayvaz, S.; Aksu, B.; Karaca, T.; Cemek, M.; Karaboga, I.; Inan, M.; Aksu, F.; Pul, M. Desferrioxamine reduces oxidative stress in the lung contusion. Sci. World J. 2013, 2013, 376959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Perez, B.E.; Gonzalez-Rojas, J.A.; Salazar, M.I.; Torres-Torres, C.; Castrejon-Jimenez, N.S. Taming the Autophagy as a Strategy for Treating COVID-19. Cells 2020, 9, 2679. [Google Scholar] [CrossRef]
- Rahman, M.; Sobur, M.; Islam, M.; Ievy, S.; Hossain, M.; El Zowalaty, M.E.; Rahman, A.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Fonkwo, P.N. Pricing infectious disease: The economic and health implications of infectious diseases. EMBO Rep. 2008, 9, S13–S17. [Google Scholar] [CrossRef] [Green Version]
- Gates, D.J. The Chlorine Dioxide Handbook; American Water Works Association: Denver, CO, USA, 1998. [Google Scholar]
- Sanekata, T.; Fukuda, T.; Miura, T.; Morino, H.; Lee, C.; Maeda, K.; Araki, K.; Otake, T.; Kawahata, T.; Shibata, T. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci. 2010, 15, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Li, X.; Hu, J.; Fan, X.; Hu, X.; Wu, G.; Xing, Y. Effect of Gaseous Chlorine Dioxide Treatment on the Quality Characteristics of Buckwheat-Based Composite Flour and Storage Stability of Fresh Noodles. Processes 2021, 9, 1522. [Google Scholar] [CrossRef]
- Jeon, Y.-A.; Lee, S.; Lee, Y.; Lee, H.-S.; Sung, J.; Kim, Y.-G. Disinfection of Fusarium-infected rice seeds by gaseous chlorine dioxide. Seed Sci. Technol. 2014, 42, 322–331. [Google Scholar] [CrossRef]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. Evaluation of chlorine dioxide gas residues on selected food produce. J. Food Sci. 2011, 76, T11–T15. [Google Scholar] [CrossRef]
- Yuk, H.G.; Bartz, J.A.; Schneider, K.R. The effectiveness of sanitizer treatments in inactivation of Salmonella spp. from bell pepper, cucumber, and strawberry. J. Food Sci. 2006, 71, M95–M99. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, J.; Kim, J.-e.; Yang, J.-y. Effect of Gaseous Chlorine Dioxide on Sterilization in Industrial Food-holding Cabinets. J. Food Hyg. Saf. 2019, 34, 170–177. [Google Scholar] [CrossRef]
- Guo, T.; Qiao, S.; Ren, T.; Tian, L.; Tian, J.; Wei, H.; Li, D.; Liu, Y.; Chen, C. Development of a chlorine dioxide air sterilizer and observation on its bactericidal efficacy. Chin. J. Disinfect. 2010, 5, 542–543. [Google Scholar]
- Hsu, C.-S.; Lu, M.-C.; Huang, D.-J. Disinfection of indoor air microorganisms in stack room of university library using gaseous chlorine dioxide. Environ. Monit. Assess. 2015, 187, 17. [Google Scholar] [CrossRef]
- Hsu, C.S.; Lu, M.C.; Huang, D.J. Effect of gaseous chlorine dioxide on student cafeteria bioaerosols. CLEAN–Soil Air Water 2014, 42, 12–19. [Google Scholar] [CrossRef]
- Burton, N.C.; Adhikari, A.; Iossifova, Y.; Grinshpun, S.A.; Reponen, T. Effect of gaseous chlorine dioxide on indoor microbial contaminants. J. Air Waste Manag. Assoc. 2008, 58, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Luftman, H.S.; Regits, M.A.; Lorcheim, P.; Czarneski, M.A.; Boyle, T.; Aceto, H.; Dallap, B.; Munro, D.; Faylor, K. Chlorine dioxide gas decontamination of large animal hospital intensive and neonatal care units. Appl. Biosaf. 2006, 11, 144–154. [Google Scholar] [CrossRef]
- Lorcheim, P. Chlorine Dioxide Gas Decontamination of a 65,000 Cubic Foot Surgical Barrier Facility. ALN Magazine (4 December 2013). Available online: http://www.alnmag.com/articles/2013/12/chlorine-dioxide-gas-decontamination-65000-cubic-foot-surgical-barrier-facility (accessed on 6 January 2014).
- Taylor, J.; Wohlers, D.; Amata, R. Toxicological Profile for Chlorine Dioxide and Chlorite; U.S. Environmental protection Agency: Washington, DC, USA, 2004.
- Fernandez, C.; Inclan, I. Chlorine and inorganic compounds. In Encyclopedia of Occupational Health and Safety, 3rd ed.; Parmeggiani, L., Ed.; International Labour Office: Geneva, Swizerland, 1983; pp. 454–457. [Google Scholar]
- Amoore, J.E.; Hautala, E. Odor as an ald to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 1983, 3, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.; MacMahon, J.; Synan, J.F. The use of chlorine dioxide in water treatment. Am. J. Public Health Nations Health 1946, 36, 1035–1037. [Google Scholar] [CrossRef] [Green Version]
- Ogata, N.; Miura, T. Inhibition of the binding of spike protein of SARS-CoV-2 coronavirus to human angiotensin-converting enzyme 2 by chlorine dioxide. Ann. Pharmacol. Pharm. 2020, 5, 1195. [Google Scholar]
- Li, J.W.; Xin, Z.T.; Wang, X.W.; Zheng, J.L.; Chao, F.H. Mechanisms of inactivation of hepatitis A virus by chlorine. Appl. Environ. Microbiol. 2002, 68, 4951–4955. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, G.B. McGraw-Hill Encyclopedia of Science & Technology: An international reference work in 20 volumes including an index. McGraw-Hill, New York, NY, 2002. Figures, tables, 90 color plates. xiii+ 15,320 pp, hardcover, 22.4 × 28.3 cm. $2,495.00. ISBN 0-07-913665-6. Chem. Educ. 2002, 7, 243–245. [Google Scholar]
- Abdel-Rahman, M.; Couri, D.; Bull, R. Kinetics of Cl02 and effects of Cl02, Cl02-, and Cl03-in drinking water on blood glutathione and hemolysis in rat and chicken. J. Environ. Pathol. Toxicol. 1979, 3, 431–449. [Google Scholar] [PubMed]
- Zart, A.; Arts, J.H.; ten Berge, W.F.; Appelman, L.M. Alternative acute inhalation toxicity testing by determination of the concentration-time-mortality relationship: Experimental comparison with standard LC50 testing. Regul. Toxicol. Pharmacol. 1992, 15, 278–290. [Google Scholar] [CrossRef]





| Group (M & F) | Real-Time ClO2 Concentration (ppm) | Dr.CLOTM Stick Numbers | Conversion of Stick Number/m3 |
|---|---|---|---|
| Control | not treated | 0 | 0 |
| Chamber #1 (n = 10) | 240< | 35~37 | ≒ (1.45~1.53) × 106 |
| Chamber #2 (n = 10) | 200~240 | 21~36 | ≒ (8.68~14.88) × 105 |
| Chamber #3 (n = 10) | 150~200 | 18~20 | ≒ (7.44~8.27) × 105 |
| Chamber #4 (n = 10) | 100~150 | 10~19 | ≒ (4.13~7.85) × 105 |
| Chamber #5 (n = 10) | 50~100 | 5~11 | ≒ (2.07~4.55) × 105 |
| Chamber #6 (n = 10) | 20~50 | 2~6 | ≒ (8.27~24.80) × 104 |
| Histopathological Examination of Lung | |
|---|---|
| Alveolar cell edema/Intra-alveolar infiltration/Congestion/ Hemorrhage/Alveolar wall disruption | Scale from 0 to 3 |
| 0 = absence of pathology (<5% maximum pathology) | |
| 1 = mild (<10%) | |
| 2 = moderate (15–20%) | |
| 3 = severe (20–25%) | |
| Leukocyte infiltration | Severity of inflammation resulting from contusions |
| 0 = no extravascular leukocytes | |
| 1 = <10 leukocytes | |
| 2 = 10~45 leukocytes | |
| 3 = 45< leukocytes | |
| M | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D10 | D11 | D12 | D13 | D14 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| *C-#1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| C-#2 | 6 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| C-#3 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| C-#4 | 3 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| C-#5 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| C-#6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| F | |||||||||||||||
| NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C-#1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| C-#2 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| C-#3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| C-#4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| C-#5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| C-#6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | Alveolar Edema | Intra-Alveolar Infiltration | Congestion | Alveolar Hemorrhage | Disruption | Total Mean Score | Leukocyte Infiltration |
|---|---|---|---|---|---|---|---|
| NT | 0.30 ± 0.153 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.06 ± 0.060 | 0.60 ± 0.306 |
| C-#1 | 1.44 ± 0.242 b,i | 1.89 ± 0.200 a,e | 2.00 ± 0.289 a,i | 1.67 ± 0.333 a,i | 1.11 ± 0.200 a | 1.62 ± 0.160 a | 2.44 ± 0.242 a,d |
| C-#2 | 1.30 ± 0.213 b | 1.20 ± 0.133 a | 1.90 ± 0.233 a,g | 1.70 ± 0.260 a,e | 0.80 ± 0.249a | 1.38 ± 0.193 c | 1.90 ± 0.277 a,e |
| C-#3 | 1.17 ± 0.307 b | 1.33 ± 0.333 a | 1.00 ± 0.447 a,d | 1.17 ± 0.477 a,d | 1.50 ± 0.563 b | 1.23 ± 0.085 c | 1.50 ± 0.342 a |
| C-#4 | 1.00 ± 0.149 b | 1.40 ± 0.163 a | 1.20 ± 0.249 a,h | 1.80 ± 0.291 b | 1.50 ± 0.269 b | 1.38 ± 0.136 c | 1.70 ± 0.213 b |
| C-#5 | 1.90 ± 0.314 c | 2.10 ± 0.233 a | 1.70 ± 0.423 a | 2.30 ± 0.335 a | 2.10 ± 0.348 a | 2.02 ± 0.102 c | 2.40 ± 0.163 a |
| C-#6 | 0.50 ± 0.189 a | 1.38 ± 0.183 a | 0.63 ± 0.263 c | 0.63 ± 0.263 a | 1.38 ± 0.324 a | 0.90 ± 0.196 c | 1.50 ± 0.189 b |
| F | Alveolar Edema | Intra-Alveolar Infiltration | Congestion | Alveolar Hemorrhage | Disruption | Total Mean Score | Leukocyte Infiltration |
|---|---|---|---|---|---|---|---|
| NT | 0.20 ± 0.133 | 0.00 ± 0.000 | 0.10 ± 0.100 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.06 ± 0.040 | 0.20 ± 0.133 |
| C-#1 | 1.90 ± 0.314 b,f,h | 2.00 ± 0.258 b,f | 2.00 ± 0.258 a,h | 2.10 ± 0.233 a,f,h | 1.90 ± 0.233 a | 1.98 ± 0.037 c | 2.30 ± 0.153 a,d |
| C-#2 | 1.56 ± 0.338 b | 1.56 ± 0.176 b | 2.22 ± 0.278 a,g | 2.67 ± 0.167 a,d,g | 1.33 ± 0.289 a | 1.88 ± 0.252 c | 2.33 ± 0.167 a,e |
| C-#3 | 1.20 ± 0.200 b | 1.40 ± 0.221 c | 1.90 ± 0.379 c | 2.40 ± 0.306 a,e,g | 1.30 ± 0.396 a | 1.64 ± 0.225 c | 2.00 ± 0.298 a |
| C-#4 | 1.60 ± 0.245 b | 1.80 ± 0.200 a | 1.40 ± 0.600 a | 1.80 ± 0.583 b | 2.00 ± 0.632 a | 1.72 ± 0.102 c | 1.80 ± 0.200 b |
| C-#5 | 0.89 ± 0.261 c | 1.00 ± 0.167 b | 0.33 ± 0.236 b | 1.33 ± 0.236 a | 2.00 ± 0.333 a | 1.10 ± 0.277 c | 1.44 ± 0.176 a |
| C-#6 | 0.80 ± 0.249 | 1.30 ± 0.260 c | 0.30 ± 0.213 c | 1.30 ± 0.153 a | 2.00 ± 0.258 a | 1.14 ± 0.284 c | 1.60 ± 0.340 b |
| Group (M) | Area Percentage (%) | Group (F) | Area Percentage (%) |
|---|---|---|---|
| Non-treated | 58.17 ± 2.003 | Non-treated | 59.35 ± 2.451 |
| Chamber #1 | 42.22 ± 3.411 a | Chamber #1 | 44.24 ± 3.241 b |
| Chamber #2 | 43.11 ± 1.791 a | Chamber #2 | 45.14 ± 2.984 b |
| Chamber #3 | 42.90 ± 1.835 a | Chamber #3 | 45.55 ± 2.225 b |
| Chamber #4 | 43.44 ± 2.495 a | Chamber #4 | 48.25 ± 2.332 b |
| Chamber #5 | 44.78 ± 3.427 c | Chamber #5 | 52.44 ± 2.551 |
| Chamber #6 | 52.40 ± 2.604 | Chamber #6 | 55.32 ± 2.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-S.; Kim, K.-M.; Boonpraman, N.; Yoon, S.-M.; Seo, J.-E.; Park, M.-W.; Moon, J.-S.; Yoo, S.-Y.; Yi, S.-S. Respiratory Safety Evaluation in Mice and Inhibition of Adenoviral Amplification in Human Bronchial Endothelial Cells Using a Novel Type of Chlorine Dioxide Gas Reactor. Toxics 2022, 10, 38. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10010038
Yang H-S, Kim K-M, Boonpraman N, Yoon S-M, Seo J-E, Park M-W, Moon J-S, Yoo S-Y, Yi S-S. Respiratory Safety Evaluation in Mice and Inhibition of Adenoviral Amplification in Human Bronchial Endothelial Cells Using a Novel Type of Chlorine Dioxide Gas Reactor. Toxics. 2022; 10(1):38. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10010038
Chicago/Turabian StyleYang, Hae-Sung, Kyeong-Min Kim, Napissara Boonpraman, Sun-Mi Yoon, Jeong-Eun Seo, Min-Woo Park, Jong-Seok Moon, Su-Young Yoo, and Sun-Shin Yi. 2022. "Respiratory Safety Evaluation in Mice and Inhibition of Adenoviral Amplification in Human Bronchial Endothelial Cells Using a Novel Type of Chlorine Dioxide Gas Reactor" Toxics 10, no. 1: 38. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10010038