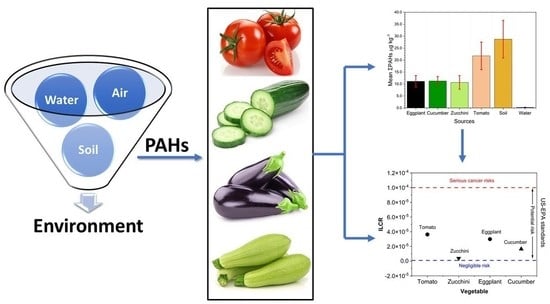
Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Area
2.3. Sampling
2.4. Sample Preparation and PAH Extraction
2.4.1. Sample Pretreatment
2.4.2. Extraction of PAHs
2.5. Instrumental Analysis
2.6. Quality Assurance and Quality Control
2.7. Estimates of Cancer Risk from PAHs
2.8. Data Analysis
3. Results and Discussion
3.1. PAHs in Irrigation Water
3.2. PAHs in Soil
3.3. PAHs in Vegetables
3.4. Transfer of PAHs
3.5. PAH Source Identification
3.5.1. Determination of PAH Sources Using Diagnostic Ratios
3.5.2. Source Identification by PCA
3.6. PAH Bioconcentration in Vegetables
3.7. The Incremental Lifetime Cancer Risk from PAHs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Famiyeh, L.; Chen, K.; Xu, J.; Sun, Y.; Guo, Q.; Wang, C.; Lv, J.; Tang, Y.-T.; Yu, H.; Snape, C.; et al. A review on analysis methods, source identification, and cancer risk evaluation of atmospheric polycyclic aromatic hydrocarbons. Sci. Total Environ. 2021, 789, 147741. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, S.; Zhou, S.; Shi, Y.; Song, J. Characteristics and source identification of polycyclic aromatic hydrocarbons (PAHs) in urban soils: A review. Pedosphere 2017, 27, 17–26. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, R.; Bhattacharya, P.; Devanesan, S.; AlSalhi, M.S. Concentration, source apportionment and potential carcinogenic risks of polycyclic aromatic hydrocarbons (PAHs) in roadside soils. Chemosphere 2022, 292, 133413. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Wang, J.; Lu, Y.; Xu, Z. Emission of PAHs, PCBs, PBDEs and heavy metals in air, water and soil around a waste plastic recycling factory in an industrial park, Eastern China. Chemosphere 2022, 294, 133734. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Villanueva, F.; Martín, P.; Salgado, S.; Moreno, A.; Sánchez-Verdú, P. Investigation of PAHs, nitrated PAHs and oxygenated PAHs in PM10 urban aerosols. A comprehensive data analysis. Chemosphere 2022, 294, 133745. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, B.; Siatecka, A.; Jędruchniewicz, K.; Sochacka, A.; Bogusz, A.; Oleszczuk, P. Polycyclic aromatic hydrocarbons (PAHs) persistence, bioavailability and toxicity in sewage sludge- or sewage sludge-derived biochar-amended soil. Sci. Total Environ. 2020, 747, 141123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, C.; Feng, Z.; Hao, Z.; Yu, W.; Wang, T.; Zou, X. Polycyclic aromatic hydrocarbons (PAHs) in marine organisms from two fishing grounds, South Yellow Sea, China: Bioaccumulation and human health risk assessment. Mar. Pollut. Bull. 2020, 153, 110995. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Bao, H.; Li, J.; Li, J.; Xing, W.; Hong, H.; Wu, F. Dynamic distribution and accumulation of PAHs in winter wheat during whole plant growth: Field investigation. Ecotoxicol. Environ. Saf. 2020, 202, 110886. [Google Scholar] [CrossRef] [PubMed]
- Douben, P.E.T. PAHs: An Ecotoxicological Perspective; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Al-Madanat, O.; AlSalka, Y.; Curti, M.; Dillert, R.; Bahnemann, D.W. Mechanistic Insights into Hydrogen Evolution by Photocatalytic Reforming of Naphthalene. ACS Catal. 2020, 10, 7398–7412. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Curti, M.; Günnemann, C.; AlSalka, Y.; Dillert, R.; Bahnemann, D.W. TiO2 photocatalysis: Impact of the platinum loading method on reductive and oxidative half-reactions. Catal. Today 2021, 380, 3–15. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Dillert, R.; Bahnemann, D.W. Photocatalytic H2 Production from Naphthalene by Various TiO2 Photocatalysts: Impact of Pt Loading and Formation of Intermediates. Catalysts 2021, 11, 107. [Google Scholar] [CrossRef]
- AlSalka, Y.; Al-Madanat, O.; Hakki, A.; Bahnemann, D.W. Boosting the H2 Production Efficiency via Photocatalytic Organic Reforming: The Role of Additional Hole Scavenging System. Catalysts 2021, 11, 1423. [Google Scholar] [CrossRef]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Wild, S.R.; Jones, K.C. Polynuclear Aromatic Hydrocarbon Uptake by Carrots Grown in Sludge-Amended Soil. J. Environ. Qual. 1992, 21, 217–225. [Google Scholar] [CrossRef]
- Kwon, H.-O.; Choi, S.-D. Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci. Total Environ. 2014, 470–471, 1494–1501. [Google Scholar] [CrossRef]
- Sverdrup, L.E.; Nielsen, T.; Krogh, P.H. Soil Ecotoxicity of Polycyclic Aromatic Hydrocarbons in Relation to Soil Sorption, Lipophilicity, and Water Solubility. Environ. Sci. Technol. 2002, 36, 2429–2435. [Google Scholar] [CrossRef]
- Meudec, A.; Dussauze, J.; Deslandes, E.; Poupart, N. Evidence for bioaccumulation of PAHs within internal shoot tissues by a halophytic plant artificially exposed to petroleum-polluted sediments. Chemosphere 2006, 65, 474–481. [Google Scholar] [CrossRef]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-Root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef]
- Bahrami, S.; Moore, F.; Keshavarzi, B. Evaluation, source apportionment and health risk assessment of heavy metal and polycyclic aromatic hydrocarbons in soil and vegetable of Ahvaz metropolis. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 71–100. [Google Scholar] [CrossRef]
- Mansour, S.A.; Belal, M.H.; Abou-Arab, A.A.K.; Gad, M.F. Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 2009, 75, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W.; Salam, A. Polycyclic aromatic hydrocarbons (PAHs) in vegetables and fruits produced in Saudi Arabia. Bull. Environ. Contam. Toxicol. 2012, 88, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Kalteh, S.; Rastkari, N.; Shamsipour, M.; Alimohammadi, M.; Yunesian, M. Health risk assessment of polycyclic aromatic hydrocarbons via dietary intake of leafy vegetables. Int. J. Environ. Anal. Chem. 2020, 1–16. [Google Scholar] [CrossRef]
- Tusher, T.R.; Sarker, M.E.; Nasrin, S.; Kormoker, T.; Proshad, R.; Islam, M.S.; Mamun, S.A.; Tareq, A.R.M. Contamination of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in rooftop vegetables and human health risks in Bangladesh. Toxin Rev. 2021, 40, 736–751. [Google Scholar] [CrossRef]
- Li, H.; Zhu, D.; Lu, X.; Du, H.; Guan, S.; Chen, Z. Determination and risk assessment of sixteen polycyclic aromatic hydrocarbons in vegetables. J. Environ. Sci. Health Part A 2018, 53, 116–123. [Google Scholar] [CrossRef]
- Al Nasir, F.; Batarseh, M.I. Agricultural reuse of reclaimed water and uptake of organic compounds: Pilot study at Mutah University wastewater treatment plant, Jordan. Chemosphere 2008, 72, 1203–1214. [Google Scholar] [CrossRef]
- Paris, A.; Ledauphin, J.; Poinot, P.; Gaillard, J.-L. Polycyclic aromatic hydrocarbons in fruits and vegetables: Origin, analysis, and occurrence. Environ. Pollut. 2018, 234, 96–106. [Google Scholar] [CrossRef]
- Zohair, A.; Salim, A.-B.; Soyibo, A.A.; Beck, A.J. Residues of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and organochlorine pesticides in organically-farmed vegetables. Chemosphere 2006, 63, 541–553. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Zhang, J.; Jin, X.; Chen, Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: Sources, exposure, and cancer risk. Environ. Pollut. 2018, 241, 750–758. [Google Scholar] [CrossRef]
- Wu, M.; Xia, Z.; Zhang, Q.; Yin, J.; Zhou, Y.; Yang, H. Distribution and Health Risk Assessment on Dietary Exposure of Polycyclic Aromatic Hydrocarbons in Vegetables in Nanjing, China. J. Chem. 2016, 2016, 1581253. [Google Scholar] [CrossRef]
- Tuteja, G.; Rout, C.; Bishnoi, N.R. Quantification of Polycyclic Aromatic Hydrocarbons in Leafy and Underground Vegetables: A Case Study Around Panipat City, Haryana, India. J. Environ. Sci. Technol. 2011, 4, 611–620. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Polycyclic Aromatic Hydrocarbons in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 1–114. [Google Scholar] [CrossRef]
- Jánská, M.; Hajšlová, J.; Tomaniová, M.; Kocourek, V.; Vávrová, M. Polycyclic Aromatic Hydrocarbons in Fruits and Vegetables Grown in the Czech Republic. Bull. Environ. Contam. Toxicol. 2006, 77, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, M.I. Polynuclear Aromatic Hydrocarbons (PAH) and Heavy Metals in Dry and Wet Sludge from As-Samra Wastewater Treatment Plant, Jordan. Soil Sediment Contam. Int. J. 2011, 20, 535–549. [Google Scholar] [CrossRef]
- Tahboub, Y.R.; Zaater, M.F.; Khater, D.F. Semi-volatile organic pollutants in Jordanian surface water. Arab. J. Chem. 2017, 10, S3318–S3323. [Google Scholar] [CrossRef] [Green Version]
- Department of Statistics; Jordanian Statistics Press: Amman, Jordan, 2021.
- AlRabadi, G.; Al-Nasir, F.; Jiries, A.; Al-Dmour, R.; Madanat, O.; Al-Dalain, S. Polychlorinated Biphenyls Residue in Citrus and Vegetables in the Jordan Valley, Jordan. Jordan J. Earth Environ. Sci. 2019, 10, 247–251. [Google Scholar]
- Jiries, A.; Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.; El Fels, L.; Mayyas, A.; Al-Dmour, R.; Al-Madanat, O.Y. Polycyclic aromatic hydrocarbons in citrus fruit irrigated with fresh water under arid conditions: Concentrations, sources, and risk assessment. Arab. J. Chem. 2022, 15, 104027. [Google Scholar] [CrossRef]
- European Commission (EC). Commission directive 2002/63/EC of 11 July 2002 establishing Community methods of sampling for the official control of pesticides in and on product of plant and animal origin and repealing Directive. Off. J. Eur. Communities 2002, 187, 703–743. [Google Scholar]
- Pang, J.; Yuan, D.; Huang, X. On-line combining monolith-based in-tube solid phase microextraction and high-performance liquid chromatography- fluorescence detection for the sensitive monitoring of polycyclic aromatic hydrocarbons in complex samples. J. Chromatogr. A 2018, 1571, 29–37. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.Á. A Practical guide to analytical method validation, including mea-surement uncertainty and accuracy profiles. Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Khalili, F.; Shariatifar, N.; Dehghani, M.H.; Yaghmaeian, K.; Nodehi, R.N.; Yaseri, M. The Analysis and Probabilistic Health Risk Assessment of Polycyclic Aromatic Hydrocarbons Contamination in Vegetables and Fruits Samples Marketed Tehran With Chemometric Approach. In Global NEST Journal; Department of Epidemiology and Biostatistics, School of Public Health: Hong Kong, China, 2020; Volume 23, pp. 497–508. [Google Scholar]
- Gratz, S.R.; Ciolino, L.A.; Mohrhaus, A.S.; Gamble, B.M.; Gracie, J.M.; Jackson, D.S.; Roetting, J.P.; McCauley, H.A.; Heitkemper, D.T.; Fricke, F.L.; et al. Screening and Determination of Polycyclic Aromatic Hydrocarbons in Seafoods Using QuEChERS-Based Extraction and High-Performance Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2011, 94, 1601–1616. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Toxicological Review of Benzo[a]pyrene: CASRN 50-32-8; Integrated Risk Information System (IRIS): Washington, DC, USA, 2017.
- Wang, N.; Lang, Y. Ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soils of Liaohe Estuarine Wetland. In Proceedings of the Conference on Environmental Pollution and Public Health, Wuhan, China, 10–12 September 2010. [Google Scholar]
- Maliszewska-Kordybach, B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl. Geochem. 1996, 11, 121–127. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Interim Canadian Environmental Quality Criteria for Contaminated Sites; CCME: Winnipeg, MB, Canada, 1991. [Google Scholar]
- Agarwal, T.; Khillare, P.S.; Shridhar, V.; Ray, S. Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. J. Hazard. Mater. 2009, 163, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.-Y.; Mo, C.-H.; Li, Y.-H.; Zeng, Q.-Y.; Katsoyiannis, A.; Wu, Q.-T.; Férard, J.-F. Occurrence and assessment of polycyclic aromatic hydrocarbons in soils from vegetable fields of the Pearl River Delta, South China. Chemosphere 2007, 68, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, X.; Zhao, J.; Sun, J. Distribution, transfer, and health risks of polycyclic aromatic hydrocarbons (PAHs) in soil-wheat systems of Henan Province, a typical agriculture province of China. Environ. Sci. Pollut. Res. 2017, 24, 18195–18203. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-D.; Shunthirasingham, C.; Daly, G.L.; Xiao, H.; Lei, Y.D.; Wania, F. Levels of polycyclic aromatic hydrocarbons in Canadian mountain air and soil are controlled by proximity to roads. Environ. Pollut. 2009, 157, 3199–3206. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, M. Some polycyclic aromatic hydrocarbons in vegetables from Northern China. J. Environ. Sci. Health Part A 2002, 37, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, W.; Xu, L.; Wu, W.; Sun, B.; Fan, W.; Zheng, H.; Huang, J. Environmental Risk Assessment of Polycyclic Aromatic Hydrocarbons in Farmland Soils near Highways: A Case Study of Guangzhou, China. Int. J. Environ. Res. Public Health 2022, 19, 10265. [Google Scholar] [CrossRef]
- Rojo Camargo, M.C.; Toledo, M.C.l.F. Polycyclic aromatic hydrocarbons in Brazilian vegetables and fruits. Food Control 2003, 14, 49–53. [Google Scholar] [CrossRef]
- Inam, E.; Ibanga, F.; Essien, J. Bioaccumulation and cancer risk of polycyclic aromatic hydrocarbons in leafy vegetables grown in soils within automobile repair complex and environ in Uyo, Nigeria. Environ. Monit. Assess. 2016, 188, 681. [Google Scholar] [CrossRef]
- Limmer, M.A.; Burken, J.G. Plant Translocation of Organic Compounds: Molecular and Physicochemical Predictors. Environ. Sci. Technol. Lett. 2014, 1, 156–161. [Google Scholar] [CrossRef]
- Jones, K.C. Organic Contaminants in the Environment: Environmental Pathways & Effects; Elsevier Applied Science: New York, NY, USA, 1991. [Google Scholar]
- Li, Y.T.; Li, F.B.; Chen, J.J.; Yang, G.Y.; Wan, H.F.; Zhang, T.B.; Zeng, X.D.; Liu, J.M. The concentrations, distribution and sources of PAHs in agricultural soils and vegetables from Shunde, Guangdong, China. Environ. Monit. Assess. 2008, 139, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Venkataraman, C. Atmospheric polycyclic aromatic hydrocarbons in Mumbai, India. Atmos. Environ. 2000, 34, 2785–2790. [Google Scholar] [CrossRef]
- Kakareka, S.V.; Kukharchyk, T.I.; Khomich, V.S. Study of PAH emission from the solid fuels combustion in residential furnaces. Environ. Pollut. 2005, 133, 383–387. [Google Scholar] [CrossRef]
- Kakareka, S.V.; Kukharchyk, T.I. PAH emission from the open burning of agricultural debris. Sci. Total Environ. 2003, 308, 257–261. [Google Scholar] [CrossRef]
- Yuan, H.; Li, T.; Ding, X.; Zhao, G.; Ye, S. Distribution, sources and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in surface soils of the Yellow River Delta, China. Mar. Pollut. Bull. 2014, 83, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Masclet, P.; Mouvier, G.; Nikolaou, K. Relative decay index and sources of polycyclic aromatic hydrocarbons. Atmos. Environ. 1986, 20, 439–446. [Google Scholar] [CrossRef]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Alagić, S.Č.; Maluckov, B.S.; Radojičić, V.B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Policy 2015, 17, 597–614. [Google Scholar] [CrossRef]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, G.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef]
- Trapp, S.; Legind, C.N. Uptake of Organic Contaminants from Soil into Vegetables and Fruits. In Dealing with Contaminated Sites: From Theory towards Practical Application; Swartjes, F.A., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 369–408. [Google Scholar] [CrossRef]
- Aamir, M.; Yin, S.; Liu, Y.; Ullah, H.; Khan, S.; Liu, W. Dietary exposure and cancer risk assessment of the Pakistani population exposed to polycyclic aromatic hydrocarbons. Sci. Total Environ. 2021, 757, 143828. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liang, Y.; Zhao, B.; Wang, Y.; Xing, F.; Qin, L. Polycyclic aromatic hydrocarbon (PAHs) geographical distribution in China and their source, risk assessment analysis. Environ. Pollut. 2019, 251, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, X.; Du, Y.-e.; Niu, X.; Guo, F.; Li, W. Cancer risk assessment of soils contaminated by polycyclic aromatic hydrocarbons in Shanxi, China. Ecotoxicol. Environ. Saf. 2019, 182, 109381. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Shi, J.; Duan, X.; Wang, B.; Huang, N.; Zhao, X. Health risk assessment of dietary exposure to polycyclic aromatic hydrocarbons in Taiyuan, China. J. Environ. Sci. 2014, 26, 432–439. [Google Scholar] [CrossRef]
- USEPA. Soil Screening Guidance: Technical Background Document; US Environmental Protection Agency: Washington, DC, USA, 1996.
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]







| Target Compound | Abb. | Mean Eggplant n = 10 | SD | % Det | Mean Tomato n = 9 | SD | % Det | Mean Zucchini. n = 9 | SD | % Det | Mean Cucumber n = 9 | SD | % Det | Mean Soil n = 18 | SD | % Det | Mean Water n = 7 | SD | % Det |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naphthalene | NAP | 0.094 | 0.083 | 20 | 0.287 | 0.243 | 78 | 1.453 | - | 11 | 0.690 | 0.672 | 22 | 2.276 | 0.894 | 100 | BQL | - | |
| Acenaphthylene | ACY | 0.586 | 0.133 | 20 | 0.335 | 0.187 | 22 | BQL | - | -- | BQL | - | -- | 1.879 | 1.295 | 39 | BQL | - | -- |
| Acenaphthene | ACE | 0.408 | 0.461 | 20 | 1.570 | 0.507 | 44 | BQL | - | -- | 0.708 | 0.359 | 33 | 4.350 | 1.565 | 28 | BQL | - | |
| Fluorene | FLO | 0.111 | - | 10 | BQL | - | -- | BQL | - | -- | 0.472 | - | 11 | 0.404 | 0.556 | 50 | BQL | - | -- |
| Phenanthrene | PHE | 0.018 | 0.009 | 30 | 1.040 | 1.395 | 22 | 0.017 | - | 44 | 0.014 | 0.001 | 22 | 0.007 | - | 6 | BQL | - | -- |
| Anthracene | ANT | 1.533 | - | 10 | 6.119 | - | 11 | 1.335 | 0.681 | 44 | 1.164 | - | 11 | 4.869 | 3.169 | 33 | BQL | - | -- |
| Fluoranthene | FLA | 0.812 | 0.227 | 100 | 0.986 | 0.470 | 89 | 0.838 | 0.339 | 100 | 0.656 | 0.170 | 89 | 0.918 | 0.546 | 100 | 0.019 | 0.005 | 43 |
| Pyrene | PYR | 0.036 | 0.013 | 70 | 0.194 | 0.231 | 100 | 0.037 | 0.036 | 100 | 0.045 | 0.0170 | 89 | 0.491 | 0.273 | 100 | 0.002 | 0.001 | 43 |
| Benzo[a]anthracene | BaA | 3.236 | 1.014 | 100 | 3.718 | 2.173 | 89 | 2.641 | 0.629 | 100 | 2.807 | 0.500 | 100 | 3.059 | 1.072 | 100 | 0.066 | 0.016 | 71 |
| Chrysene | CHR | 0.039 | 0.035 | 50 | 0.137 | 0.133 | 56 | 0.116 | - | 11 | BQL | - | -- | 0.182 | 0.120 | 94 | 0.002 | 0.001 | 43 |
| Benzo[b]fluoranthene | BbF | 0.516 | 0.105 | 90 | 0.776 | 0.517 | 100 | 0.342 | 0.095 | 100 | 0.441 | 0.261 | 100 | 2.330 | 0.162 | 94 | 0.013 | 0.003 | 100 |
| Benzo[k]fluoranthene | BkF | 0.017 | 0.009 | 90 | 0.280 | 0.533 | 44 | 0.013 | 0.009 | 33 | 0.009 | 0.003 | 44 | 0.071 | 0.096 | 22 | 0.001 | 0.001 | 57 |
| Benzo[a]pyrene | BaP | 3.234 | 1.268 | 100 | 5.436 | 2.127 | 100 | 3.333 | 1.429 | 100 | 3.471 | 1.077 | 100 | 6.776 | 2.780 | 100 | 0.096 | 0.039 | 100 |
| Indeno[1.2.3.4.cd]pyrene | IcdP | 0.179 | 0.063 | 80 | 0.384 | 0.272 | 78 | 0.207 | 0.094 | 100 | 0.251 | 0.203 | 100 | 0.414 | 0.224 | 67 | 0.010 | 0.004 | 43 |
| Dibenzo[a.h]anthracene | DahA | 0.014 | 0.020 | 20 | 0.178 | 0.194 | 33 | 0.077 | 0.023 | 33 | 0.176 | 0.146 | 44 | 0.382 | 0.477 | 39 | 0.002 | - | 14 |
| Benzo[g.h.i]perylene | BghiP | 0.286 | 0.142 | 90 | 0.337 | 0.337 | 100 | 0.240 | 0.082 | 100 | 0.353 | 0.336 | 100 | 0.316 | 0.375 | 94 | 0.009 | 0.005 | 100 |
| ∑PAHs | 11.120 | 21.774 | 10.649 | 11.256 | 28.724 | 0.218 | |||||||||||||
| ∑COMB | 8.355 | 12.247 | 7.767 | 8.032 | 14.557 | - | |||||||||||||
| ∑COMB/∑PAHs | 0.751 | 0.562 | 0.729 | 0.714 | 0.507 | - | |||||||||||||
| ∑LMW PAHs | 2.751 | 9.350 | 2.805 | 3.048 | 13.785 | - | |||||||||||||
| ∑HMW PAHs | 8.369 | 12.424 | 7.844 | 8.208 | 14.939 | - | |||||||||||||
| ∑LMW/∑HMW | 0.329 | 0.753 | 0.358 | 0.371 | 0.923 | - | |||||||||||||
| BaP/BghiP | 11.292 | 16.149 | 13.897 | 9.828 | 21.464 | - | |||||||||||||
| BaP/(BaP + CHY) | 0.988 | 0.975 | 0.966 | 1.000 | 0.974 | - | |||||||||||||
| BaA/(BaA + CHR) | 0.988 | 0.965 | 0.958 | 1.000 | 0.944 | - | |||||||||||||
| FLA/PYR | 22.332 | 5.071 | 22.556 | 14.749 | 1.870 | - | |||||||||||||
| ANT/(ANT + PHE) | 0.988 | 0.855 | 0.987 | 0.988 | 0.999 | - | |||||||||||||
| FL/(FL + PYR) | 0.957 | 0.835 | 0.958 | 0.937 | 0.652 | - |
| Diagnostic Ratio | Value | Source Apportionment |
|---|---|---|
| ANT/(ANT + PHE) | <0.1 | petroleum (petrogenic source) |
| >0.1 | combustion (pyrogenic sources) | |
| FLA/(FLA + PYR) | <0.4 | petrogenic |
| 0.4–0.5 | liquid fossil fuel burning | |
| >0.5 | coal, grass, and wood burning | |
| BaA/(BaA + CHR) | <0.2 | petroleum (petrogenic source) |
| 0.2–0.35 | fuel combustion (vehicular emissions) | |
| >0.35 | coal, grass, and wood burning | |
| Bap/(Bap + CHR) | <0.20 | petrogenic origin |
| 0.2 −0.35 | vehicular and combustion source | |
| >0.35 | coal, wood, and grass burning source | |
| BaP/BghiP | >0.6 | traffic emissions |
| <0.6 | nontraffic emissions | |
| FLA/PYR | <1 | petrogenic source |
| >1 | pyrogenic source | |
| LMW/HMW | <1.0 | pyrogenic sources such as coal, grass, and burning of wood |
| >1.0 | petrogenic sources, such as fuel or one refined petroleum product | |
| ∑comb/∑16PAHs | ~1 | combustion |
| 16 PAHs | # of Rings | BCF Cucumber | BCF Eggplant | BCF Zucchini | BCF Tomato |
|---|---|---|---|---|---|
| NAP | 2 | 0.54 | 0.07 | 1.14 | 0.22 |
| ACY | 3 | ND | 0.31 | ND | 0.18 |
| ACE | 3 | 0.19 | 0.11 | ND | 0.43 |
| FLO | 3 | 1.79 | 0.42 | ND | ND |
| PHE | 3 | 4.14 | 5.35 | 4.98 | 303.15 |
| ANT | 3 | 0.24 | 0.32 | 0.28 | 1.26 |
| FLA | 4 | 0.71 | 0.88 | 0.91 | 1.07 |
| PYR | 4 | 0.09 | 0.07 | 0.08 | 0.40 |
| BaA | 4 | 0.92 | 1.06 | 0.86 | 1.22 |
| CHR | 4 | ND | 0.22 | 0.64 | 0.75 |
| BbF | 5 | 1.16 | 1.35 | 0.90 | 2.03 |
| BkF | 5 | 0.17 | 0.32 | 0.25 | 5.38 |
| BaP | 5 | 0.51 | 0.48 | 0.49 | 0.80 |
| IcdP | 6 | 0.65 | 0.46 | 0.54 | 0.99 |
| DahA | 6 | 0.55 | 0.05 | 0.24 | 0.56 |
| BghiP | 6 | 1.17 | 0.95 | 0.79 | 1.11 |
| ∑16 PAHs | 0.45 | 0.45 | 0.43 | 0.88 | |
| ∑LMW-PAHs | 0.26 | 0.23 | 0.24 | 0.79 | |
| ∑HMW-PAHs | 0.64 | 0.65 | 0.61 | 0.97 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.M.; Jiries, A.; Al-Madanat, O.Y.; Mayyas, A.; A. Al-Dalain, S.; Al-Dmour, R.; Alahmad, A.; Batarseh, M.I. Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables. Toxics 2022, 10, 643. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10110643
Al-Nasir F, Hijazin TJ, Al-Alawi MM, Jiries A, Al-Madanat OY, Mayyas A, A. Al-Dalain S, Al-Dmour R, Alahmad A, Batarseh MI. Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables. Toxics. 2022; 10(11):643. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10110643
Chicago/Turabian StyleAl-Nasir, Farh, Tahani J. Hijazin, Mutaz M. Al-Alawi, Anwar Jiries, Osama Y. Al-Madanat, Amal Mayyas, Saddam A. Al-Dalain, Rasha Al-Dmour, Abdalrahim Alahmad, and Mufeed I. Batarseh. 2022. "Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables" Toxics 10, no. 11: 643. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10110643