Uptake, Elimination and Effects of Cosmetic Microbeads on the Freshwater Gastropod Biomphalaria glabrata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Microbeads from Facial Scrub
2.2. Quantification of Microbeads
2.3. Identification of Microbeads
2.4. Digestion Method
2.5. Test Organism
2.6. Experimental Design
2.7. Short-Term (48-h) Exposure
2.8. Long-Term Exposure (21 d)
2.9. Statistics
3. Results and Discussions
3.1. Shape, Size and Chemical Composition of Microbeads in Facial Scrub
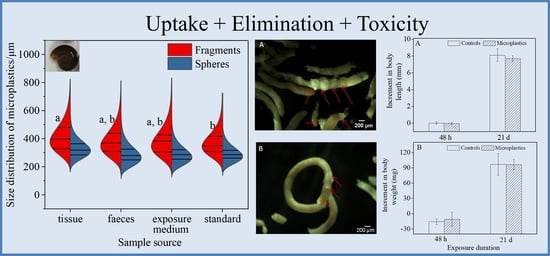
3.2. Uptake and Elimination of Microbeads in Short-Term and Long-Term Assays
3.2.1. Short-Term Exposure Assay
3.2.2. Long-Term Exposure Assay
3.3. In Vivo Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Eriksen, M.; Liboiron, M.; Kiessling, T.; Charron, L.; Alling, A.; Lebreton, L.; Richards, H.; Roth, B.; Ory, N.C.; Hidalgo-Ruz, V.; et al. Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 2018, 232, 430–439. [Google Scholar] [CrossRef]
- Naidoo, T.; Glassom, D.; Smit, A.J. Plastic pollution in five urban estuaries of KwaZulu-Natal, South Africa. Mar. Pollut. Bull. 2015, 101, 473–480. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Peng, G.; Bellerby, R.; Zhang, F.; Sun, X.; Li, D. The ocean’s ultimate trashcan: Hadal trenches as major depositories for plastic pollution. Water Res. 2020, 168, 115121. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kross, S.M.; Armstrong, J.B.; Bogan, M.T.; Darling, E.S.; Green, S.J.; Smyth, A.R.; Verissimo, D. Scientific evidence supports a ban on microbeads. Environ. Sci. Technol. 2015, 49, 10759–10761. [Google Scholar] [CrossRef] [Green Version]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, Y.; Liangwei, W.; Liang, J.; Liu, T.; Zhu, M.; Li, Q.; Sun, X. Characteristics of microplastics ingested by zooplankton from the Bohai Sea, China. Sci. Total. Environ. 2020, 713, 136357. [Google Scholar] [CrossRef]
- Guven, O.; Gokdag, K.; Jovanovic, B.; Kideys, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Desforges, J.P.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef]
- Peters, C.A.; Thomas, P.A.; Rieper, K.B.; Bratton, S.P. Foraging preferences influence microplastic ingestion by six marine fish species from the Texas Gulf Coast. Mar. Pollut. Bull. 2017, 124, 82–88. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in aquatic ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Proc. Natl. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef] [Green Version]
- Weltje, L.; Sumpter, J.P. What makes a concentration environmentally relevant? Critique and a proposal. Environ. Sci. Technol. 2017, 51, 11520–11521. [Google Scholar] [CrossRef]
- Chisada, S.; Yoshida, M.; Karita, K. Polyethylene microbeads are more critically toxic to the eyes and reproduction than the kidneys or growth in medaka, Oryzias latipes. Environ. Pollut. 2021, 268, 115957. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Kuehnel, D.; Puntar, B.; Gotvajn, A.Ž.; Kalčikova, G. An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Grigorakis, S.; Mason, S.A.; Drouillard, K.G. Determination of the gut retention of plastic microbeads and microfibers in goldfish (Carassius auratus). Chemosphere 2017, 169, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Impact of microplastic beads and fibers on waterflea (Ceriodaphnia dubia) survival, growth, and reproduction: Implications of single and mixture exposures. Environ. Sci. Technol. 2017, 51, 13397–13406. [Google Scholar] [CrossRef] [Green Version]
- Imhof, H.K.; Laforsch, C. Hazardous or not—Are adult and juvenile individuals of Potamopyrgus antipodarum affected by non-buoyant microplastic particles? Environ. Pollut. 2016, 218, 383–391. [Google Scholar] [CrossRef]
- Weber, A.; von Randow, M.; Voigt, A.L.; von der Au, M.; Fischer, E.; Meermann, B.; Wagner, M. Ingestion and toxicity of microplastics in the freshwater gastropod Lymnaea stagnalis: No microplastic-induced effects alone or in combination with copper. Chemosphere 2021, 263, 128040. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.; Le Goic, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Baynes, A.; Lockyer, A.E.; Routledge, E.J.; Jones, C.S.; Noble, L.R.; Jobling, S. Steroid androgen exposure during development has no effect on reproductive physiology of biomphalaria glabrata. PLoS ONE 2016, 11, e0159852. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.C.; Caixeta, M.B.; Araujo, P.S.; Goncalves, B.B.; Araujo, O.A.; Silva, L.D.; Rocha, T.L. Gonadal histopathology and inflammatory response in the freshwater snail exposed to iron oxide nanoparticles and ferric chloride: Insights into reproductive nanotoxicity. Aquat. Toxicol. 2021, 237, 105910. [Google Scholar] [CrossRef]
- Dubaish, F.; Liebezeit, G. Suspended microplastics and black carbon particles in the Jade system, Southern North Sea. Water Air Soil Pollut. 2013, 224, 1352. [Google Scholar] [CrossRef]
- Lozano, R.L.; Mouat, J. Marine Litter in the North-East Atlantic Region: Assessment and Priorities for Response; KIMO International: Shetland, UK, 2009. [Google Scholar]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Cole, M. A novel method for preparing microplastic fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef] [Green Version]
- Renner, K.O.; Foster, H.A.; Routledge, E.J.; Scrimshaw, M.D. A comparison of different approaches for characterizing microplastics in selected personal care products. Environ. Toxicol. Chem. 2021, 00, 1–8. [Google Scholar] [CrossRef]
- Dawson, A.; Huston, W.; Kawaguchi, S.; King, C.; Cropp, R.; Wild, S.; Eisenmann, P.; Townsend, K.; Bengtson Nash, S. Uptake and Depuration Kinetics Influence microplastic bioaccumulation and toxicity in antarctic krill (Euphausia superba). Environ. Sci. Technol. 2018, 52, 3195–3201. [Google Scholar] [CrossRef]
- Panebianco, A.; Nalbone, L.; Giarratana, F.; Ziino, G. First discoveries of microplastics in terrestrial snails. Food Control 2019, 106, 106722. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Wang, X.; Bolan, N.; Tsang, D.C.W.; Sarkar, B.; Bradney, L.; Li, Y. A review of microplastics aggregation in aquatic environment: Influence factors, analytical methods, and environmental implications. J. Hazard. Mater. 2021, 402, 123496. [Google Scholar] [CrossRef]
- Yokota, K.; Waterfield, H.; Hastings, C.; Davidson, E.; Kwietniewski, E.; Wells, B. Finding the missing piece of the aquatic plastic pollution puzzle: Interaction between primary producers and microplastics. Limnol. Oceanogr. 2017, 2, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef]
- Coppock, R.L.; Galloway, T.S.; Cole, M.; Fileman, E.S.; Queiros, A.M.; Lindeque, P.K. Microplastics alter feeding selectivity and faecal density in the copepod, Calanus helgolandicus. Sci. Total Environ. 2019, 687, 780–789. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.K.; Fileman, E.; Clark, J.; Lewis, C.; Halsband, C.; Galloway, T.S. Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol. 2016, 50, 3239–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, P.; Singdahl-Larsen, C.; Lusher, A.L. Understanding the occurrence and fate of microplastics in coastal Arctic ecosystems: The case of surface waters, sediments and walrus (Odobenus rosmarus). Sci. Total Environ. 2021, 792, 148308. [Google Scholar] [CrossRef] [PubMed]
- Van Colen, C.; Moereels, L.; Vanhove, B.; Vrielinck, H.; Moens, T. The biological plastic pump: Evidence from a local case study using blue mussel and infaunal benthic communities. Environ. Pollut. 2021, 274, 115825. [Google Scholar] [CrossRef]
- Munuera, P.; Salvat-Leal, I.; Belmonte, A.; Romero, D. Can microplastics influence the accumulation of Pb in tissues of blue crab? Int. J. Environ. Res. Public Health 2021, 18, 3599. [Google Scholar] [CrossRef] [PubMed]
- Kaposi, K.L.; Mos, B.; Kelaher, B.P.; Dworjanyn, S.A. Ingestion of microplastic has limited impact on a marine larva. Environ. Sci. Technol. 2014, 48, 1638–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalcikova, G.; Skalar, T.; Marolt, G.; Jemec Kokalj, A. An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res. 2020, 175, 115644. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Baynes, A.; Renner, K.O.; Zhang, M.; Scrimshaw, M.D.; Routledge, E.J. Uptake, Elimination and Effects of Cosmetic Microbeads on the Freshwater Gastropod Biomphalaria glabrata. Toxics 2022, 10, 87. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10020087
Wang Y, Baynes A, Renner KO, Zhang M, Scrimshaw MD, Routledge EJ. Uptake, Elimination and Effects of Cosmetic Microbeads on the Freshwater Gastropod Biomphalaria glabrata. Toxics. 2022; 10(2):87. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10020087
Chicago/Turabian StyleWang, Ying, Alice Baynes, Kofi O. Renner, Mingxing Zhang, Mark D. Scrimshaw, and Edwin J. Routledge. 2022. "Uptake, Elimination and Effects of Cosmetic Microbeads on the Freshwater Gastropod Biomphalaria glabrata" Toxics 10, no. 2: 87. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10020087