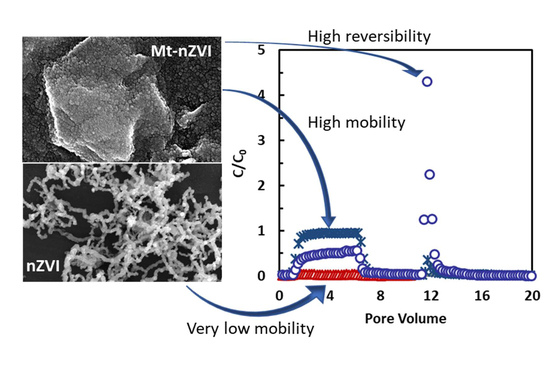
Significant Mobility of Novel Heteroaggregates of Montmorillonite Microparticles with Nanoscale Zerovalent Irons in Saturated Porous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of nZVIs and Heteroaggregates of Mt-nZVIs
2.2. Porous Media
2.3. Transport Experiments
2.4. Calculation of DLVO Interaction Energies
3. Results and Discussion
3.1. Characteristics of Particles and Collector Grains
3.2. Particle Deposition in Sand Porous Media
3.3. Particle Release in Sand Porous Media
3.4. Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-B.; Zhang, W.X. Synthesizing nanoscale iron particles for rapid and complete dichlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025. [Google Scholar] [CrossRef]
- Schrick, B.; Hydutsky, B.W.; Blough, J.L.; Mallouk, T.E. Delivery vehicles for zerovalent metal nanoparticles in soil and groundwater. Chem. Mater. 2004, 16, 2187–2193. [Google Scholar] [CrossRef]
- Johnson, R.L.; Nurmi, J.T.; O’Brien Johnson, G.S.; Fan, D.; O’Brien Johnson, R.L.; Shi, Z.; Salter-Blanc, A.J.; Tratnyek, P.G.; Lowry, G.V. Field-scale transport and transformation of carboxymethylcellulose-stabilized nano zero-valent iron. Environ. Sci. Technol. 2013, 47, 1573–1580. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Lowry, G.V. Sulfidized nanoscale zero-valent iron: Tuning the properties of this complex material for efficient groundwater remediation. Acc. Mater. Res. 2021, 2, 420–431. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Xu, N.; Yang, L.; Jing, P.; Chen, J. Enhanced transport and chromium remediation of nano-zero valent iron modified by tea polyphenol extracts and carboxymethyl cellulose in water-soil media. J. Soils Sediments 2022, 22, 196–207. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D. Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res. 2007, 41, 2101–2108. [Google Scholar] [CrossRef]
- Shen, C.; Jin, Y.; Zhuang, J.; Li, T.; Xing, B. Role and importance of surface heterogeneities in transport of particles in saturated porous media. Crit. Rev. Environ. Sci. Technol. 2020, 50, 244–329. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Jia, H.; Li, H.; Teppen, B.J.; Boyd, S.A. Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates. Environ. Sci. Technol. 2010, 44, 4258–5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Chen, H.; Zhang, M.; Cao, X. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 2014, 152, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shen, C.; Bi, X.; Li, T. Removal of hexavalent chromium from aqueous solution by fabricating novel heteroaggregates of montmorillonite microparticles with nanoscale zero-valent iron. Sci. Rep. 2020, 10, 12137. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; O’Melia, C.R. Kinetics of deposition of colloidal particles in porous media. Environ. Sci. Technol. 1990, 24, 1528–1536. [Google Scholar] [CrossRef]
- Tufenkji, N.; Elimelech, M. Breakdown of colloid filtration theory: Role of the secondary energy minimum and surface charge heterogeneities. Langmuir 2005, 21, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shen, C.; Wu, S.; Jin, C.; Bradford, S.A. Synergies of surface roughness and hydration on colloid detachment in saturated porous media: Column and atomic force microscopy studies. Water Res. 2020, 183, 116068. [Google Scholar] [CrossRef]
- Shen, C.; Haque, M.E.; Wang, D.; Zheng, W.; Yin, Y.; Huang, Y. Observed equilibrium partition and second-order kinetic interaction of quantum dot nanoparticles in saturated porous media. J. Contam. Hydrol. 2021, 240, 103799. [Google Scholar] [CrossRef]
- Hahn, M.W.; Abadzic, D.; O’Melia, C.R. Aquasols: On the role of secondary minima. Environ. Sci. Technol. 2004, 38, 5915–5924. [Google Scholar] [CrossRef]
- Shen, C.; Li, B.; Huang, Y.; Jin, Y. Kinetics of coupled primary- and secondary-minimum deposition of colloids under unfavorable chemical conditions. Environ. Sci. Technol. 2007, 41, 6976–6982. [Google Scholar] [CrossRef]
- Torkzaban, S.; Kim, H.N.; Simunek, J.; Bradford, S.A. Hysteresis of colloid retention and release in saturated porous media during transients in solution chemistry. Environ. Sci. Technol. 2010, 44, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, L.-P.; Li, B.; Huang, Y.; Jin, Y. Role of surface roughness in chemical detachment of colloids deposited at primary energy minima. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Basnet, M.; Ghoshal, S.; Tufenkji, N. Rhamnolipid biosurfactant and soy protein act as effective stabilizers in the aggregation and transport of palladium-doped zerovalent iron nanoparticles in saturated porous media. Environ. Sci. Technol. 2013, 47, 13355–13364. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pedel, J.; Fife, P.; Johnson, W.P. Hemispheres-in-cell geometry to predict colloid deposition in porous media. Environ. Sci. Technol. 2009, 43, 8573–8579. [Google Scholar] [CrossRef]
- Tufenkji, N.; Elimelech, M. Deviation from the classical colloid filtration theory in the presence of repulsive DLVO interactions. Langmuir 2004, 20, 10818–10828. [Google Scholar] [CrossRef]
- Shen, C.; Wang, H.; Lazouskaya, V.; Du, Y.; Lu, W.; Wu, J.; Zhang, H.; Huang, Y. Cotransport of bismerthiazol and montmorillonite colloids in saturated porous media. J. Contam. Hydrol. 2015, 177–178, 18–29. [Google Scholar] [CrossRef]
- Du, Y.; Bradford, S.A.; Shen, C.; Li, T.; Bi, X.; Liu, D.; Huang, Y. Novel analytical expressions for determining van der Waals interaction between a particle and air-water interface: Unexpected stronger van der Waals force than capillary force. J. Colloid Interface Sci. 2022, 610, 982–993. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Elimelech, M. Surface element integration: A novel technique for evaluation of DLVO interaction between a particle and flat plate. J. Colloid Interface Sci. 1997, 193, 273–285. [Google Scholar] [CrossRef]
- Shen, C.; Wu, L.; Zhang, S.; Ye, H.; Li, B.; Huang, Y. Heteroaggregation of microparticles with nanoparticles changes the chemical reversibility of the microparticles’ attachment to planar surfaces. J. Colloid Interface Sci. 2014, 421, 103–113. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London-van der Waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual coagulation of colloidal dispersion. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Oliveira, R. Understanding adhesion: A means for preventing fouling. Exp. Therm. Fluid Sci. 1997, 14, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Hoek, E.M.V.; Agarwal, G.K. Extended DLVO interactions between spherical particles and rough surfaces. J. Colloid Interface Sci. 2006, 298, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bobba, A.D.; Attinti, R.; Shen, C.; Lazouskaya, V.; Wang, L.-P.; Jin, Y. Retention and transport of silica nanoparticles in saturated porous media: Effect of concentration and particle size. Environ. Sci. Technol. 2012, 46, 7151–7158. [Google Scholar] [CrossRef]
- Shen, C.; Bradford, S.A.; Li, T.; Li, B.; Huang, Y. Can nanoscale surface charge heterogeneity really explain colloid detachment from primary minima upon reductio of solution ionic strength? J. Nanopart. Res. 2018, 20, 165. [Google Scholar] [CrossRef]
- Li, T.; Shen, C.; Johnson, W.P.; Ma, H.; Jin, C.; Zhang, C.; Chu, X.; Ma, K.; Xing, B. Important role of concave surfaces in deposition of colloids under favorable conditions as revealed by microscale visualization. Environ. Sci. Technol. 2022, 56, 4121–4131. [Google Scholar] [CrossRef]
- Lin, S.; Wiesner, M.R. Deposition of aggregated nanoparticles-A theoretical and experimental study on the effect of aggregation state on the affinity between nanoparticles and a collector surface. Environ. Sci. Technol. 2012, 46, 13270–13277. [Google Scholar] [CrossRef]
- Cai, L.; Tong, M.; Wang, X.; Kim, H. Influence of clay particles on the transport and retention of titanium dioxide nanoparticles in quartz sand. Environ. Sci. Technol. 2014, 48, 7323–7332. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Lu, T.; Chen, J.; Wei, Q.; Chen, W.; Zhou, Y.; Qi, Z. Colloid-mediated transport of tetracycline in saturated porous media: Comparison between ferrihydrite and montmorillonite. J. Environ. Manag. 2021, 299, 113638. [Google Scholar] [CrossRef]
- Zhan, J.; Zheng, T.; Piringer, G.; Day, C.; McPherson, G.L.; Lu, Y.; Papadopoulos, K.; John, V.T. Transport characteristics of nanoscale functional zerovalent iron/silica composites for in-situ remediation of trichloroethylene. Environ. Sci. Technol. 2008, 42, 8871–8876. [Google Scholar] [CrossRef]
- Vecchia, E.D.; Luna, M.; Sethi, R. Transport in porous media of highly concentrated iron micro- and nanoparticles in the presence of xanthan gum. Environ. Sci. Technol. 2009, 43, 8942–8947. [Google Scholar] [CrossRef] [PubMed]
- Kocur, C.M.; Chowdhury, A.I.; Sakulchaicharoen, N.; Boparai, H.K.; Weber, K.P.; Sharma, P.; Krol, M.M.; Austrins, L.; Peace, C.; Sleep, B.E.; et al. Characterization of nZVI mobility in a field scale test. Environ. Sci. Technol. 2014, 48, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, M.; Li, X. Improved longevity of nanoscale zero-valent iron with a magnesium hydroxide coating shell for the removal of Cr(VI) in sand columns. Environ. Int. 2019, 133, 105249. [Google Scholar] [CrossRef] [PubMed]
- Monga, Y.; Kumar, P.; Sharma, R.K.; Filip, J.; Varma, R.S.; Zboril, R.; Gawande, M.B. Sustainable synthesis of nanoscale zerovalent iron particles for environmental remediation. ChemSusChem 2020, 13, 3288–3305. [Google Scholar] [CrossRef]
- Bradford, S.A.; Yates, S.R.; Bettahar, M.; Simunek, J. Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resour. Res. 2002, 38, 1327. [Google Scholar] [CrossRef] [Green Version]
- Bradford, S.A.; Simunek, J.; Bettahar, M.; van Genuchten, M.T.; Yates, S.A. Significance of straining in colloid deposition: Evidence and implications. Water Resour. Res. 2006, 42, W12S15. [Google Scholar] [CrossRef]
- Auset, M.; Keller, A.A. Pore-scale visualization of colloid straining and filtration in saturated porous media using micromodels. Water Resour. Res. 2006, 42, W12S02. [Google Scholar] [CrossRef] [Green Version]
- Porubcan, A.A.; Xu, S. Colloid straining within saturated heterogeneous porous media. Water Resour. 2011, 45, 1796–1806. [Google Scholar] [CrossRef]
- Shang, W.; Morales, V.L.; Zhang, W.; Stoof, C.R.; Gao, B.; Schatz, A.L.; Zhang, Y.; Steenhuis, T.S. Quantification of colloid retention and release by straining and energy minima in variably saturated porous media. Environ. Sci. Technol. 2013, 47, 8256–8264. [Google Scholar]
- Hahn, M.W.; O’Melia, C.R. Deposition and reentrainment of Brownian particles in porous media under unfavorable chemical conditions: Some concepts and applications. Environ. Sci. Technol. 2004, 38, 210–220. [Google Scholar] [CrossRef]
- Shi, K.; Wang, X.; Chu, X.; Li, T.; Yin, Y.; Shen, C. Impact of heavy metal cations on deposition and release of clay colloids in saturated porous media. Vadose Zone J. 2022, e20179. [Google Scholar] [CrossRef]
- Li, T.; Jin, Y.; Huang, Y.; Li, B.; Shen, C. Observed dependence of colloid detachment on the concentration of initially attached colloids and collector surface heterogeneity in porous media. Environ. Sci. Technol. 2017, 51, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V.; Syngouna, V.I. Attachment of bacteriophages MS2 and ΦX174 onto kaolinite and montmorillonite: Extended DLVO interactions. Colloids Surf. B 2012, 92, 74–83. [Google Scholar] [CrossRef]
- Seymour, M.B.; Chen, G.; Su, C.; Li, Y. Transport and retention of colloids in porous media: Does shape really matter? Environ. Sci. Technol. 2013, 47, 8391–8398. [Google Scholar] [CrossRef] [PubMed]
- Fountouli, T.V.; Chrysikopoulos, C.V. Effect of clay colloid particles on formaldehyde transport in unsaturated porous media. Water 2020, 12, 3541. [Google Scholar] [CrossRef]
- Ma, H.; Bolster, C.; Johnson, W.P.; Li, K.; Pazmino, E.; Camacho, K.M.; Anselmo, A.C.; Mitragotri, S. Coupled influences of particle shape, surface property and flow hydrodynamics on rod-shaped colloid transport in porous media. J. Colloid Interface Sci. 2020, 577, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bhattacharjee, S.; Hoek, E.M. Is surface roughness a “scapegoat” or a primary factor when defining particle-substrate interaction. Langmuir 2010, 26, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Tosco, T.; Bosch, J.; Meckenstock, R.U.; Sethi, R. Transport of ferrihydrite nanoparticles in saturated porous media: Role of ionic strength and flow rate. Environ. Sci. Technol. 2012, 46, 4008–4015. [Google Scholar] [CrossRef] [Green Version]
- Redman, J.A.; Walker, S.L.; Elimelech, M. Bacterial adhesion and transport in porous media: Role of the secondary energy minimum. Environ. Sci Technol. 2004, 38, 1777–1785. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zeng, S.; Shen, C.; Jin, C.; Huang, Y. Interactions between nanoparticles and fractal surfaces. Water Res. 2019, 151, 296–309. [Google Scholar] [CrossRef]
- Bizmark, N.; Schneider, J.; Priestley, R.D.; Datta, S.S. Multiscale dynamics of colloidal deposition and erosion in porous media. Sci. Adv. 2020, 6, eabc2530. [Google Scholar] [CrossRef] [PubMed]
- Nishad, S.; Al-Raoush, R.I.; Alazaiza, M.Y.D. Release of colloids in saturated porous media under transient hydro-chemical conditions: A pore-scale study. Colloids Surf. A 2021, 614, 126188. [Google Scholar] [CrossRef]
- Parvan, A.; Jafari, S.; Rahnama, M.; Norouzi-Apourvari, S.; Raoof, A. Insight into particle detachment in clogging of porous media; a pore scale study using lattice Boltzmann method. Adv. Water Resour. 2021, 151, 103888. [Google Scholar] [CrossRef]
- De Vries, E.T.; Tang, Q.; Faez, S.; Raoof, A. Fluid flow and colloid transport experiment in singe-porosity sample; tracking of colloid transport behavior in a saturated micromodel. Adv. Water Resour. 2022, 159, 104086. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Teng, J.; Zheng, W.; Liu, D.; Ma, K. Significant Mobility of Novel Heteroaggregates of Montmorillonite Microparticles with Nanoscale Zerovalent Irons in Saturated Porous Media. Toxics 2022, 10, 332. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10060332
Shen C, Teng J, Zheng W, Liu D, Ma K. Significant Mobility of Novel Heteroaggregates of Montmorillonite Microparticles with Nanoscale Zerovalent Irons in Saturated Porous Media. Toxics. 2022; 10(6):332. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10060332
Chicago/Turabian StyleShen, Chongyang, Jinan Teng, Wenjuan Zheng, Dong Liu, and Ke Ma. 2022. "Significant Mobility of Novel Heteroaggregates of Montmorillonite Microparticles with Nanoscale Zerovalent Irons in Saturated Porous Media" Toxics 10, no. 6: 332. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10060332