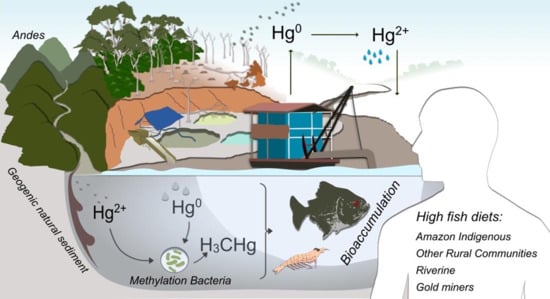
Trends in Mercury Contamination Distribution among Human and Animal Populations in the Amazon Region
Abstract
:1. Introduction
2. Material and Methods
- Population: Human and animal population in the Amazon region.
- Exposure: Exposure to mercury (Hg).
- Comparator: Different levels of mercury exposure or comparisons between different species or regions, and the values allowed by health organizations.
- Outcome: Hg contamination in animal tissues and humans relative to diet.
- Study Characteristics: DOI, title, and author(s) of the study.
- Population Characteristics: Whether the study focuses on human or non-human species, the scientific nomenclature of the studied species, and their feeding guild.
- Collection Details: Sample collection location (in decimal degrees) and the period during which the samples were collected.
- Mercury Exposure Details: Type of tissue sampled and the total amount of mercury found in these tissues.
- Additional Data: Any mention of indigenous regions, hydroelectric dams, or artisanal and small-scale gold mining (ASGM), as well as details of mercury analysis.
2.1. Geoprocessing
2.2. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Overview of THg Levels in Animal Research
3.3. Overview of THg Levels in Human Research
3.4. Mining
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paiva, P.F.P.R.; de Lourdes Pinheiro Ruivo, M.; da Silva Júnior, O.M.; de Nazaré Martins Maciel, M.; Braga, T.G.M.; de Andrade, M.M.N.; dos Santos Junior, P.C.; da Rocha, E.S.; de Freitas, T.P.M.; da Silva Leite, T.V.; et al. Deforestation in Protect Areas in the Amazon: A Threat to Biodiversity. Biodivers. Conserv. 2020, 29, 19–38. [Google Scholar] [CrossRef]
- Garcia, B. The Amazon from an International Law Perspective; Cambridge University Press: New York, NY, USA, 2011; ISBN 1-139-49668-9. [Google Scholar]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia Is the Primary Source of Neotropical Biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Berenguer, E.; França, F.; Ferreira, J.; Lees, A.C.; Louzada, J.; Sayer, E.J.; Solar, R.; Smith, C.C.; Aragão, L.E.O.C.; et al. Linking Land-Use and Land-Cover Transitions to Their Ecological Impact in the Amazon. Proc. Natl. Acad. Sci. USA 2022, 119, e2202310119. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-Term Forest Degradation Surpasses Deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Giljum, S.; Maus, V.; Kuschnig, N.; Luckeneder, S.; Tost, M.; Sonter, L.J.; Bebbington, A.J. A Pantropical Assessment of Deforestation Caused by Industrial Mining. Proc. Natl. Acad. Sci. USA 2022, 119, e2118273119. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Carnaval, A.C.; Flantua, S.G.A.; Lohmann, L.G.; Ribas, C.C.; Riff, D.; Carrillo, J.D.; Fan, Y.; Figueiredo, J.J.P.; Guayasamin, J.M.; et al. Human Impacts Outpace Natural Processes in the Amazon. Science 2023, 379, eabo5003. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Orellana, J.; Oliveira, M.; Hacon, S.; Basta, P. Human Mercury Exposure in Yanomami Indigenous Villages from the Brazilian Amazon. IJERPH 2018, 15, 1051. [Google Scholar] [CrossRef]
- Siqueira-Gay, J.; Sánchez, L.E. The Outbreak of Illegal Gold Mining in the Brazilian Amazon Boosts Deforestation. Reg. Environ. Chang. 2021, 21, 28. [Google Scholar] [CrossRef]
- Achatz, R.W.; De Vasconcellos, A.C.S.; Pereira, L.; Viana, P.V.D.S.; Basta, P.C. Impacts of the Goldmining and Chronic Methylmercury Exposure on the Good-Living and Mental Health of Munduruku Native Communities in the Amazon Basin. IJERPH 2021, 18, 8994. [Google Scholar] [CrossRef]
- Basta, P.C.; De Sousa Viana, P.V.; De Vasconcellos, A.C.S.; Santos Périssé, A.R.; Hofer, C.B.; Paiva, N.S.; Kempton, J.W.; De Andrade, D.C.; De Oliveira, R.A.A.; Achatz, R.; et al. Mercury Exposure in Munduruku Indigenous Communities from Brazilian Amazon: Methodological Background and an Overview of the Principal Results. Int. J. Environ. Res. Public Health 2021, 18, 9222. [Google Scholar] [CrossRef]
- Hacon, S.D.S.; Oliveira-da-Costa, M.; Gama, C.D.S.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury Exposure through Fish Consumption in Traditional Communities in the Brazilian Northern Amazon. Int. J. Environ. Res. Public Health 2020, 17, 5269. [Google Scholar] [CrossRef]
- Pestana, I.A.; De Rezende, C.E.; Almeida, R.; De Lacerda, L.D.; Bastos, W.R. Let’s Talk about Mercury Contamination in the Amazon (Again): The Case of the Floating Gold Miners’ Village on the Madeira River. Extr. Ind. Soc. 2022, 11, 101122. [Google Scholar] [CrossRef]
- Valdelamar-Villegas, J.; Olivero-Verbel, J. High Mercury Levels in the Indigenous Population of the Yaigojé Apaporis National Natural Park, Colombian Amazon. Biol. Trace Elem. Res. 2020, 194, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, A.C.S.D.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.D.M.; Lima, M.D.O.; Jesus, I.M.D.; Hacon, S.D.S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 7940. [Google Scholar] [CrossRef] [PubMed]
- Calvimontes, J.; Massaro, L.; Araujo, C.H.X.; Moraes, R.R.; Mello, J.; Ferreira, L.C.; De Theije, M. Small-Scale Gold Mining and the COVID-19 Pandemic: Conflict and Cooperation in the Brazilian Amazon. Extr. Ind. Soc. 2020, 7, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Velásquez Ramírez, M.G.; Barrantes, J.A.G.; Thomas, E.; Gamarra Miranda, L.A.; Pillaca, M.; Tello Peramas, L.D.; Bazán Tapia, L.R. Heavy Metals in Alluvial Gold Mine Spoils in the Peruvian Amazon. Catena 2020, 189, 104454. [Google Scholar] [CrossRef]
- Adler Miserendino, R.; Guimarães, J.R.D.; Schudel, G.; Ghosh, S.; Godoy, J.M.; Silbergeld, E.K.; Lees, P.S.J.; Bergquist, B.A. Mercury Pollution in Amapá, Brazil: Mercury Amalgamation in Artisanal and Small-Scale Gold Mining or Land-Cover and Land-Use Changes? ACS Earth Space Chem. 2018, 2, 441–450. [Google Scholar] [CrossRef]
- Bullock, E.L.; Woodcock, C.E.; Souza, C.; Olofsson, P. Satellite-based Estimates Reveal Widespread Forest Degradation in the Amazon. Glob. Chang. Biol. 2020, 26, 2956–2969. [Google Scholar] [CrossRef]
- Gallay, M.; Mora, A.; Martinez, J.; Gardel, A.; Laraque, A.; Sarrazin, M.; Beaucher, E.; Doudou, J.; Lagane, C. Dynamics and Fluxes of Organic Carbon and Nitrogen in Two Guiana Shield River Basins Impacted by Deforestation and Mining Activities. Hydrol. Process. 2018, 32, 17–29. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Mora-Silva, D.; D’Orio, G.; Tapia-Segarra, E.; Gaibor, I.D.; Esparza Parra, J.F.; Chávez Velásquez, C.R.; Straface, S. Artisanal and Small-Scale Gold Mining (ASGM): Management and Socioenvironmental Impacts in the Northern Amazon of Ecuador. Sustainability 2022, 14, 6854. [Google Scholar] [CrossRef]
- Veiga, M.M.; Gunson, A.J. Gravity Concentration in Artisanal Gold Mining. Minerals 2020, 10, 1026. [Google Scholar] [CrossRef]
- Dethier, E.N.; Sartain, S.L.; Lutz, D.A. Heightened Levels and Seasonal Inversion of Riverine Suspended Sediment in a Tropical Biodiversity Hot Spot Due to Artisanal Gold Mining. Proc. Natl. Acad. Sci. USA 2019, 116, 23936–23941. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem.—Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Espejo, J.C.; Messinger, M.; Román-Dañobeytia, F.; Ascorra, C.; Fernandez, L.E.; Silman, M. Deforestation and Forest Degradation Due to Gold Mining in the Peruvian Amazon: A 34-Year Perspective. Remote Sens. 2018, 10, 1903. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury Exposure Assessment in Indigenous Communities from Tarapaca Village, Cotuhe and Putumayo Rivers, Colombian Amazon. Environ. Sci. Pollut. Res. 2019, 26, 36458–36467. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C. Special Issue on Bioconversion, Bioaccumulation and Toxicity of Mercury in a Changing World. Appl. Sci. 2020, 10, 6548. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Pereira, W.V.D.S.; Souza, E.S.D.; Ramos, S.J.; Dias, Y.N.; Lima, M.W.D.; De Souza Neto, H.F.; Oliveira, E.S.D.; Fernandes, A.R. Artisanal Gold Mining in the Eastern Amazon: Environmental and Human Health Risks of Mercury from Different Mining Methods. Chemosphere 2021, 284, 131220. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Cellular Conditions Responsible for Methylmercury-Mediated Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 7218. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; de Matos Macchi, B.; do Nascimento, J.L.M.; Maia, C.S.; Lima, R.R.; Arrifano, G.P. Mercury: What Can We Learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef]
- Crespo-López, M.E.; Augusto-Oliveira, M.; Santos-Sacramento, L.; Lopes-Araújo, A. Mercúrio Na Amazônia Uma Breve Contextualizaçao Do Problema; Universidade do Estado do Amazonas: Manaus, Brazil, 2021. [Google Scholar]
- De Oliveira, D.F.; De Castro, B.S.; Do Nascimento Recktenvald, M.C.N.; Da Costa Júnior, W.A.; Da Silva, F.X.; De Menezes Alves, C.L.; Froehlich, J.D.; Bastos, W.R.; Ott, A.M.T. Mercury in Wild Animals and Fish and Health Risk for Indigenous Amazonians. Food Addit. Contam. Part B 2021, 14, 161–169. [Google Scholar] [CrossRef]
- Baturin, G.; Gordeev, V. Geochemistry of Suspended Matter in the Amazon River Waters. Geochem. Int. 2019, 57, 197–205. [Google Scholar] [CrossRef]
- Kasper, D.; Forsberg, B.R.; do Amaral Kehrig, H.; Amaral, J.H.F.; Bastos, W.R.; Malm, O. Mercury in Black-Waters of the Amazon; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-90121-3. [Google Scholar]
- Fisher, J.A.; Schneider, L.; Fostier, A.-H.; Guerrero, S.; Guimarães, J.R.D.; Labuschagne, C.; Leaner, J.J.; Martin, L.G.; Mason, R.P.; Somerset, V.; et al. A Synthesis of Mercury Research in the Southern Hemisphere, Part 2: Anthropogenic Perturbations. Ambio 2023, 52, 918–937. [Google Scholar] [CrossRef]
- Brito, B.C.; Forsberg, B.R.; Kasper, D.; Amaral, J.H.F.; De Vasconcelos, M.R.R.; De Sousa, O.P.; Cunha, F.A.G.; Bastos, W.R. The Influence of Inundation and Lake Morphometry on the Dynamics of Mercury in the Water and Plankton in an Amazon Floodplain Lake. Hydrobiologia 2017, 790, 35–48. [Google Scholar] [CrossRef]
- Salazar-Camacho, C.; Salas-Moreno, M.; Marrugo-Madrid, S.; Marrugo-Negrete, J.; Díez, S. Dietary Human Exposure to Mercury in Two Artisanal Small-Scale Gold Mining Communities of Northwestern Colombia. Environ. Int. 2017, 107, 47–54. [Google Scholar] [CrossRef]
- Diringer, S.E.; Berky, A.J.; Marani, M.; Ortiz, E.J.; Karatum, O.; Plata, D.L.; Pan, W.K.; Hsu-Kim, H. Deforestation Due to Artisanal and Small-Scale Gold Mining Exacerbates Soil and Mercury Mobilization in Madre de Dios, Peru. Environ. Sci. Technol. 2019, 54, 286–296. [Google Scholar] [CrossRef]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araújo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human Neurotoxicity of Mercury in the Amazon: A Scoping Review with Insights and Critical Considerations. Ecotoxicol. Environ. Saf. 2021, 208, 111686. [Google Scholar] [CrossRef]
- Miguel, V.M.N.; Darlan, Q. Brito Mercury (Hg) Researches in Brazilian Biomes: A Scientometric Analysis between the Years 1991 and 2018. J. Veterina Sci. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Basta, P.C.; de Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.D.O.D.E.R.; de Aguiar, D.S.; de Souza, C.C.; Oliveira-da-Costa, M. Risk Assessment of Mercury-Contaminated Fish Consumption in the Brazilian Amazon: An Ecological Study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Moulatlet, G.M.; Yacelga, N.; Rico, A.; Mora, A.; Hauser-Davis, R.A.; Cabrera, M.; Capparelli, M.V. A Systematic Review on Metal Contamination Due to Mining Activities in the Amazon Basin and Associated Environmental Hazards. Chemosphere 2023, 339, 139700. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, T.C.; Penteado, J.O.; dos Santos, M.; da Silva Júnior, F.M.R. Methylmercury in Fish from the Amazon Region—A Review Focused on Eating Habits. Water Air Soil Pollut. 2021, 232, 199. [Google Scholar] [CrossRef]
- RAISG Amazon in Numbers. Available online: https://www.raisg.org/en/infographic/ (accessed on 1 August 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Cochrane. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2019. [Google Scholar]
- Kirby, A. Exploratory Bibliometrics: Using VOSviewer as a Preliminary Research Tool. Publications 2023, 11, 10. [Google Scholar] [CrossRef]
- Cohen Hubal, E.A.; Frank, J.J.; Nachman, R.; Angrish, M.; Deziel, N.C.; Fry, M.; Tornero-Velez, R.; Kraft, A.; Lavoie, E. Advancing Systematic-Review Methodology in Exposure Science for Environmental Health Decision Making. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 906–916. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. General Standard For Contaminants And Toxins In Food And Feed CXS 193-1995. Codex Alimentarius. 2019, 1–13. [Google Scholar]
- WHO. Chapter 6.9 Mercury General Description. In Air Quality Guidelines, 2nd ed.; WHO: Geneva, Switzerland, 2000; pp. 1–15. [Google Scholar]
- Ministério da Saúde RESOLUÇÃO—RDC No 42, DE 29 DE AGOSTO DE 2013. 2013. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/anvisa-resolucao-rdc-no-42-de-29-de-agosto-de-2013-internaliza-a-resolucao-gmc-res-n-o-12-2011.pdf/view (accessed on 16 November 2023).
- Poulin, J.; Gibb, H.; Prüss-Üstün, A.; World Health Organization. Mercury: Assessing the Environmental Burden of Disease at National and Local Levels; Prüss-Üstün, A., Ed.; World Health Organization: Geneva, Switzerland, 2008.
- Costa Junior, J.M.F.; Silva, C.I.M.D.; Lima, A.A.D.S.; Rodrigues Júnior, D.; Silveira, L.C.D.L.; Souza, G.D.S.; Pinheiro, M.D.C.N. Teores de Mercúrio Em Cabelo e Consumo de Pescado de Comunidades Ribeirinhas Na Amazônia Brasileira, Região Do Tapajós. Cienc. Saude Coletiva 2018, 23, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Yao, C.; Tian, X.; Wu, Y.; Xie, Q.; Wang, D. Mercury in Human Hair and Its Implications for Health Investigation. Curr. Opin. Environ. Sci. Health 2021, 22, 100271. [Google Scholar] [CrossRef]
- QGIS Association QGIS.Org 2023. Available online: https://qgis.org/en/site/ (accessed on 16 November 2023).
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Southern America|Realm & Subrealms|One Earth. Available online: https://www.oneearth.org/realms/southern-america/ (accessed on 16 November 2023).
- Tang, L.; Werner, T.T. Author Correction: Global Mining Footprint Mapped from High-Resolution Satellite Imagery. Commun Earth Environ. 2023, 4, 163. [Google Scholar] [CrossRef]
- Yevugah, L.L.; Darko, G.; Bak, J. Does Mercury Emission from Small-Scale Gold Mining Cause Widespread Soil Pollution in Ghana? Environ. Pollut. 2021, 284, 116945. [Google Scholar] [CrossRef]
- Goix, S.; Maurice, L.; Laffont, L.; Rinaldo, R.; Lagane, C.; Chmeleff, J.; Menges, J.; Heimbürger, L.-E.; Maury-Brachet, R.; Sonke, J.E. Quantifying the Impacts of Artisanal Gold Mining on a Tropical River System Using Mercury Isotopes. Chemosphere 2019, 219, 684–694. [Google Scholar] [CrossRef]
- Gerson, J.R.; Szponar, N.; Zambrano, A.A.; Bergquist, B.; Broadbent, E.; Driscoll, C.T.; Erkenswick, G.; Evers, D.C.; Fernandez, L.E.; Hsu-Kim, H. Amazon Forests Capture High Levels of Atmospheric Mercury Pollution from Artisanal Gold Mining. Nat. Commun. 2022, 13, 559. [Google Scholar] [CrossRef]
- Feingold, B.J.; Berky, A.; Hsu-Kim, H.; Rojas Jurado, E.; Pan, W.K. Population-Based Dietary Exposure to Mercury through Fish Consumption in the Southern Peruvian Amazon. Environ. Res. 2020, 183, 108720. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Silva, S.F.D.; Oliveira, D.C.; Pereira, J.P.G.; Castro, S.P.; Costa, B.N.S.; Lima, M.D.O. Seasonal Variation of Mercury in Commercial Fishes of the Amazon Triple Frontier, Western Amazon Basin. Ecol. Indic. 2019, 106, 105549. [Google Scholar] [CrossRef]
- Ferreira Da Silva, S.; De Oliveira Lima, M. Mercury in Fish Marketed in the Amazon Triple Frontier and Health Risk Assessment. Chemosphere 2020, 248, 125989. [Google Scholar] [CrossRef]
- Souza-Araujo, J.; Souza-Junior, O.G.; Guimarães-Costa, A.; Hussey, N.E.; Lima, M.O.; Giarrizzo, T. The Consumption of Shark Meat in the Amazon Region and Its Implications for Human Health and the Marine Ecosystem. Chemosphere 2021, 265, 129132. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Sucupira, M.C.A.; Ortolani, E.L.; López-Alonso, M. Toxic and Essential Trace Element Concentrations in Fish Species in the Lower Amazon, Brazil. Sci. Total Environ. 2020, 732, 138983. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and Selenium in Fishes from the Tapajós River in the Brazilian Amazon: An Evaluation of Human Exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Biomonitoring of Mercury, Cadmium and Selenium in Fish and the Population of Puerto Nariño, at the Southern Corner of the Colombian Amazon. Arch. Environ. Contam. Toxicol. 2020, 79, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.F.; Pereira, J.P.G.; Oliveira, D.C.; Lima, M.D.O. Methylmercury in Predatory and Non-Predatory Fish Species Marketed in the Amazon Triple Frontier. Bull. Environ. Contam. Toxicol. 2020, 104, 733–737. [Google Scholar] [CrossRef] [PubMed]
- De Castro Paiva, T.; Dary, E.P.; Pestana, I.A.; Amadio, S.A.; Malm, O.; Kasper, D. Flood-Pulse and Trophic Position Modulate Mercury Concentrations in Fishes from an Amazon Floodplain Lake. Environ. Res. 2022, 215, 114307. [Google Scholar] [CrossRef] [PubMed]
- Finoto Viana, L.; Damasceno De Souza, D.C.; Batista Da Silva, E.; Kummrow, F.; Lima Cardoso, C.A.; De Lima, N.A.; Crispim, B.D.A.; Barufatti, A.; Florentino, A.C. Bioaccumulation of Metals and Genotoxic Effects in Females of Colomesus Asellus Collected in an Amazon River Estuary, Amapá, Brazil. Limnetica 2023, 42, 203–214. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Total and Methyl Mercury Distribution in Water, Sediment, Plankton and Fish along the Tapajós River Basin in the Brazilian Amazon. Chemosphere 2019, 235, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.D.; Silva, D.M.D.; Franco, T.S.B.S.; Vasconcelos, C.R.S.; Sousa, D.J.D.A.D.; Sarrazin, S.L.F.; Sakamoto, M.; Bourdineaud, J.-P. Fish Consumption Habits of Pregnant Women in Itaituba, Tapajós River Basin, Brazil: Risks of Mercury Contamination as Assessed by Measuring Total Mercury in Highly Consumed Piscivore Fish Species and in Hair of Pregnant Women. Arch. Ind. Hyg. Toxicol. 2022, 73, 131–142. [Google Scholar] [CrossRef] [PubMed]
- De Souza Azevedo, J.; Hortellani, M.A.; De Souza Sarkis, J.E. Organotropism of Total Mercury (THg) in Cichla Pinima, Ecological Aspects and Human Consumption in Fish from Amazon Region, Brazil. Environ. Sci. Pollut. Res. 2019, 26, 21363–21370. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Montes, C.; Ferreira, M.A.P.; Giarrizzo, T.; Amado, L.L.; Rocha, R.M. The Legacy of Artisanal Gold Mining and Its Impact on Fish Health from Tapajós Amazonian Region: A Multi-Biomarker Approach. Chemosphere 2022, 287, 132263. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.S.; Pestana, I.A.; Da Costa Nery, A.F.; Bastos, W.R.; Souza, C.M.M. Variation in Hg Accumulation between Demersal and Pelagic Fish from Puruzinho Lake, Brazilian Amazon. Ecotoxicology 2019, 28, 1143–1149. [Google Scholar] [CrossRef]
- Cavecci-Mendonça, B.; Cavalcante de Souza Vieira, J.; Monteiro de Lima, P.; Leite, A.L.; Buzalaf, M.A.R.; Zara, L.F.; de Magalhães Padilha, P. Study of Proteins with Mercury in Fish from the Amazon Region. Food Chem. 2020, 309, 125460. [Google Scholar] [CrossRef]
- de Queiroz, J.V.; Cavecci-Mendonça, B.; Vieira, J.C.S.; Martins, R.A.; de Almeida Assunção, A.S.; Cavallini, N.G.; dos Santos, F.A.; de Magalhães Padilha, P. Metalloproteomic Strategies for Identifying Proteins as Biomarkers of Mercury Exposure in Serrasalmus Rhombeus from the Amazon Region. Biol. Trace Elem. Res. 2021, 199, 712–720. [Google Scholar] [CrossRef]
- Soares, J.M.; Gomes, J.M.; Anjos, M.R.; Silveira, J.N.; Custódio, F.B.; Gloria, M.B.A. Mercury in Fish from the Madeira River and Health Risk to Amazonian and Riverine Populations. Food Res. Int. 2018, 109, 537–543. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Nery, A.F.D.C.; Bastos, W.R.; Souza, C.M.M. Influence of the Flood Pulse on Mercury Accumulation in Detritivorous, Herbivorous and Omnivorous Fish in Brazilian Amazonia. Ecotoxicology 2019, 28, 478–485. [Google Scholar] [CrossRef]
- Vieira, J.C.S.; De Oliveira, G.; Cavallini, N.G.; Braga, C.P.; Adamec, J.; Zara, L.F.; Buzalaf, M.A.R.; De Magalhães Padilha, P. Investigation of Protein Biomarkers and Oxidative Stress in Pinirampus Pirinampu Exposed to Mercury Species from the Madeira River, Amazon-Brazil. Biol. Trace Elem. Res. 2022, 200, 1872–1882. [Google Scholar] [CrossRef]
- Da Cunha Bataglioli, I.; De Queiroz, J.V.; Vieira, J.C.S.; Cavalline, N.G.; Braga, C.P.; Buzalaf, M.A.R.; Zara, L.F.; Adamec, J.; De Magalhães Padilha, P. Mercury Metalloproteomic Profile in Muscle Tissue of Arapaima Gigas from the Brazilian Amazon. Environ. Monit. Assess. 2022, 194, 705. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Almeida, M.G.; Ferreira Da Costa Nery, A.; Bastos, W.R.; Magalhães Souza, C.M. Mercury Biomagnification in an Ichthyic Food Chain of an Amazon Floodplain Lake (Puruzinho Lake): Influence of Seasonality and Food Chain Modeling. Ecotoxicol. Environ. Saf. 2021, 207, 111249. [Google Scholar] [CrossRef]
- Bittarello, A.C.; Vieira, J.C.S.; Braga, C.P.; De Paula Araújo, W.L.; Da Cunha Bataglioli, I.; Da Silva, J.M.; Buzalaf, M.A.R.; Fleuri, L.F.; De Magalhães Padilha, P. Characterization of Molecular Biomarkers of Mercury Exposure to Muscle Tissue of Plagioscion Squamosissimus and Colossoma Macropomum from the Amazon Region. Food Chem. 2019, 276, 247–254. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, J.V.; Vieira, J.C.S.; De Oliveira, G.; Braga, C.P.; Da Cunha Bataglioli, I.; Da Silva, J.M.; De Paula Araújo, W.L.; De Magalhães Padilha, P. Identification of Biomarkers of Mercury Contamination in Brachyplatystoma Filamentosum of the Madeira River, Brazil, Using Metalloproteomic Strategies. Biol. Trace Elem. Res. 2019, 187, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Mussy, M.H.; De Almeida, R.; De Carvalho, D.P.; Lauthartte, L.C.; De Holanda, I.B.B.; Almeida, M.G.D.; De Sousa-Filho, I.F.; De Rezende, C.E.; Malm, O.; Bastos, W.R. Evaluating Total Mercury and Methylmercury Biomagnification Using Stable Isotopes of Carbon and Nitrogen in Fish from the Madeira River Basin, Brazilian Amazon. Environ. Sci. Pollut. Res. 2022, 30, 33543–33554. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Ozório, R.O.A.; Rodriguez, A.F.R.; Faria, F.S.E.D.V.; Furtado, C.M.; Ribeiro, R.A. Mercury Distribution in Two Commercial Fish Species (Pimelodus Maculatus and Calophysus Macropterus)—Case Study of River Acre (Acre State, Brazilian Amazon). Hum. Ecol. Risk Assess. 2020, 26, 1439–1448. [Google Scholar] [CrossRef]
- Vieira, J.C.S.; Braga, C.P.; Queiroz, J.V.D.; Cavecci-Mendonça, B.; Oliveira, G.D.; Freitas, N.G.D.; Fernandes, A.A.H.; Fernandes, M.D.S.; Buzalaf, M.A.R.; Adamec, J.; et al. The Effects of Mercury Exposure on Amazonian Fishes: An Investigation of Potential Biomarkers. Chemosphere 2023, 316, 137779. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.C.S.; Braga, C.P.; De Oliveira, G.; Padilha, C.D.C.F.; De Moraes, P.M.; Zara, L.F.; Leite, A.D.L.; Buzalaf, M.A.R.; Padilha, P.D.M. Mercury Exposure: Protein Biomarkers of Mercury Exposure in Jaraqui Fish from the Amazon Region. Biol. Trace Elem. Res. 2018, 183, 164–171. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, J.V.; Vieira, J.C.S.; Da Cunha Bataglioli, I.; Bittarello, A.C.; Braga, C.P.; De Oliveira, G.; Do Carmo Federici Padilha, C.; De Magalhães Padilha, P. Total Mercury Determination in Muscle and Liver Tissue Samples from Brazilian Amazon Fish Using Slurry Sampling. Biol. Trace Elem. Res. 2018, 184, 517–522. [Google Scholar] [CrossRef]
- Vieira, J.C.S.; Cavecci, B.; Queiroz, J.V.; Braga, C.P.; Padilha, C.C.F.; Leite, A.L.; Figueiredo, W.S.; Buzalaf, M.A.R.; Zara, L.F.; Padilha, P.M. Determination of the Mercury Fraction Linked to Protein of Muscle and Liver Tissue of Tucunaré (Cichla Spp.) from the Amazon Region of Brazil. Arch. Environ. Contam. Toxicol. 2015, 69, 422–430. [Google Scholar] [CrossRef]
- da Silva Montes, C.; Pantoja Ferreira, M.A.; Giarrizzo, T.; Amado, L.L.; Rocha, R.M. Evaluation of Metal Contamination Effects in Piranhas through Biomonitoring and Multi Biomarkers Approach. Heliyon 2020, 6, e04666. [Google Scholar] [CrossRef]
- Souza-Araujo, J.; Andrades, R.; Hauser-Davis, R.A.; Lima, M.O.; Giarrizzo, T. Before the Dam: A Fish-Mercury Contamination Baseline Survey at the Xingu River, Amazon Basin Before the Belo Monte Dam. Bull. Environ. Contam. Toxicol. 2022, 108, 861–866. [Google Scholar] [CrossRef]
- De Matos, L.S.; Silva Correa, A.S.A.; Da Silva, S.A.A.; Muniz, C.C.; Alves Ignacio, A.R. Mercury Concentrations in Fish and Human Health Assessment in Preflood Phase of a Hydro Dam in Teles Pires River, Southern Brazilian Amazon. Elementa 2021, 9, 20. [Google Scholar] [CrossRef]
- Matos, L.S.D.; Silva, J.O.S.; Kasper, D.; Carvalho, L.N. Assessment of Mercury Contamination in Brycon falcatus (Characiformes: Bryconidae) and Human Health Risk by Consumption of This Fish from the Teles Pires River, Southern Amazonia. Neotrop. Ichthyol. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; De Lima, N.A.; Do Amaral Crispim, B.; Barufatti, A.; Florentino, A.C. Metal Bioaccumulation in Fish from the Araguari River (Amazon Biome) and Human Health Risks from Fish Consumption. Environ. Sci. Pollut. Res. 2023, 30, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Costa, M.; Viana, L.F.; Lima Cardoso, C.A.; Gonar Silva Isacksson, E.D.; Silva, J.C.; Florentino, A.C. Landscape Composition and Inorganic Contaminants in Water and Muscle Tissue of Plagioscion Squamosissimus in the Araguari River (Amazon, Brazil). Environ. Res. 2022, 208, 112691. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.D.D.; Nascimento, E.L.D.; Faccheti, M.S.D.A.; Nunes, N.N.D.S.; Gomes, J.P.D.O.; Almeida, R.D.; Bastos, W.R. Mercury in Muscle and Liver of Plagioscion Squamosissimus (Acanthuriformes: Sciaenidae) from the Machado River, Brazilian Amazon. Acta Amaz. 2022, 52, 60–68. [Google Scholar] [CrossRef]
- Martinez, G.; McCord, S.; Driscoll, C.; Todorova, S.; Wu, S.; Araújo, J.; Vega, C.; Fernandez, L. Mercury Contamination in Riverine Sediments and Fish Associated with Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2018, 15, 1584. [Google Scholar] [CrossRef]
- Barocas, A.; Vega, C.; Alarcon Pardo, A.; Araujo Flores, J.M.; Fernandez, L.; Groenendijk, J.; Pisconte, J.; Macdonald, D.W.; Swaisgood, R.R. Local Intensity of Artisanal Gold Mining Drives Mercury Accumulation in Neotropical Oxbow Lake Fishes. Sci. Total Environ. 2023, 886, 164024. [Google Scholar] [CrossRef]
- Rodriguez-Levy, I.E.; Van Damme, P.A.; Carvajal-Vallejos, F.M.; Bervoets, L. Trace Element Accumulation in Different Edible Fish Species from the Bolivian Amazon and the Risk for Human Consumption. Heliyon 2022, 8, e11649. [Google Scholar] [CrossRef]
- De Vasconcellos, A.C.S.; Ferreira, S.R.B.; De Sousa, C.C.; De Oliveira, M.W.; De Oliveira Lima, M.; Basta, P.C. Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics 2022, 10, 516. [Google Scholar] [CrossRef]
- Borges, A.C.; Da Silva Montes, C.; Barbosa, L.A.; Ferreira, M.A.P.; Berrêdo, J.F.; Martins Rocha, R. Integrated Use of Histological and Ultrastructural Biomarkers for Assessing Mercury Pollution in Piranhas (Serrasalmus rhombeus) from the Amazon Mining Region. Chemosphere 2018, 202, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Vreedzaam, A.; Ouboter, P.; Hindori-Mohangoo, A.D.; Lepak, R.; Rumschlag, S.; Janssen, S.; Landburg, G.; Shankar, A.; Zijlmans, W.; Lichtveld, M.Y.; et al. Contrasting Mercury Contamination Scenarios and Site Susceptibilities Confound Fish Mercury Burdens in Suriname, South America. Environ. Pollut. 2023, 336, 122447. [Google Scholar] [CrossRef] [PubMed]
- Laffont, L.; Menges, J.; Goix, S.; Gentès, S.; Maury-Brachet, R.; Sonke, J.E.; Legeay, A.; Gonzalez, P.; Rinaldo, R.; Maurice, L. Hg Concentrations and Stable Isotope Variations in Tropical Fish Species of a Gold-Mining-Impacted Watershed in French Guiana. Environ. Sci. Pollut. Res. 2021, 28, 60609–60621. [Google Scholar] [CrossRef] [PubMed]
- Montaña, C.G.; Liverpool, E.; Taphorn, D.C.; Schalk, C.M. The Cost of Gold: Mercury Contamination of Fishes in a Neotropical River Food Web. Neotrop. Ichthyol. 2021, 19, e200155. [Google Scholar] [CrossRef]
- Cunha Bataglioli, I.D.; Souza Vieira, J.C.; Vitor De Queiroz, J.; Da Silva Fernandes, M.; Bittarello, A.C.; Braga, C.P.; Rabelo Buzalaf, M.A.; Adamec, J.; Zara, L.F.; Magalhães Padilha, P.D. Physiological and Functional Aspects of Metal-Binding Protein Associated with Mercury in the Liver Tissue of Pirarucu (Arapaima gigas) from the Brazilian Amazon. Chemosphere 2019, 236, 124320. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.F.; Cardoso, C.A.L.; Lima-Junior, S.E.; Súarez, Y.R.; Florentino, A.C. Bioaccumulation of Metal in Liver Tissue of Fish in Response to Water Toxicity of the Araguari-Amazon River, Brazil. Environ. Monit. Assess. 2020, 192, 781. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Dias, S.R.; Ortolani, E.L.; López-Alonso, M. Toxic and Essential Trace Element Concentrations in the Freshwater Shrimp Macrobrachium Amazonicum in the Lower Amazon, Brazil. J. Food Compos. Anal. 2020, 86, 103361. [Google Scholar] [CrossRef]
- Pinheiro-Sousa, D.B.; da Costa Soares, S.H.; Torres, H.S.; de Jesus, W.B.; de Oliveira, S.R.S.; Bastos, W.R.; de Oliveira Ribeiro, C.A.; Carvalho-Neta, R.N.F. Sediment Contaminant Levels and Multibiomarker Approach to Assess the Health of Catfish Sciades Herzbergii in a Harbor from the Northern Brazilian Amazon. Ecotoxicol. Environ. Saf. 2021, 208, 111540. [Google Scholar] [CrossRef]
- Lopes, M.C.B.; de CARVALHO, G.O.; Bernardo, R.R.; Macedo, J.; Lino, A.S.; Ramalho, E.E.; Kasper, D.; Meire, R.O.; Torres, J.P.M.; Malm, O. Total Mercury in Wild Felids Occurring in Protected Areas in the Central Brazilian Amazon. Acta Amaz. 2020, 50, 142–148. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Carvalho, D.P.; Gravena, W.; De Almeida, R.; Mussy, M.H.; Sousa, E.A.; Holanda, I.B.B.; De Sousa-Filho, I.F.; Bastos, W.R. Total Mercury and Methylmercury in River Dolphins (Cetacea: Iniidae: Inia Spp.) in the Madeira River Basin, Western Amazon. Environ. Sci. Pollut. Res. 2021, 28, 45121–45133. [Google Scholar] [CrossRef]
- Salazar-Pammo, A.C.; Achá, D.; Miranda-Chumacero, G. Preferential Liver Accumulation of Mercury Explains Low Concentrations in Muscle of Caiman yacare (Alligatoridae) in Upper Amazon. Bull. Environ. Contam. Toxicol. 2021, 106, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.N.D.; Recktenvald, M.C.N.D.N.; de Carvalho, D.P.; Puerta, E.L.B.; de Sousa-Filho, I.F.; Dórea, J.G.; Bastos, W.R. Mercury in Birds (Aquatic and Scavenger) from the Western Amazon. Environ. Res. 2021, 201, 111574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Divoll, T.J.; Ganguli, P.M.; Trama, F.A.; Lamborg, C.H. Presence of Artisanal Gold Mining Predicts Mercury Bioaccumulation in Five Genera of Bats (Chiroptera). Environ. Pollut. 2018, 236, 862–870. [Google Scholar] [CrossRef]
- Moreno-Brush, M.; Portillo, A.; Brändel, S.D.; Storch, I.; Tschapka, M.; Biester, H. Mercury Concentrations in Bats (Chiroptera) from a Gold Mining Area in the Peruvian Amazon. Ecotoxicology 2018, 27, 45–54. [Google Scholar] [CrossRef]
- Da Silva Júnior, F.J.T.M.; Ribeiro, J.D.N.; Da Silva, H.L.A.; Da Silva Carneiro, C.; De Jesus, E.F.O.; De Araújo, U.B.; Lazzarini, S.M.; Souza, A.R.; Simões, J.S.; Lopes, R.T.; et al. Study of Inorganic Elements in Different Organs and Tissues of Amazonian Manatee (Trichechus inunguis) from Brazil. Environ. Sci. Pollut. Res. 2022, 29, 30486–30495. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Guerra, F.; Trujillo, F.; Parks, D.; Oliveira-da-Costa, M.; Van Damme, P.A.; Echeverría, A.; Franco, N.; Carvajal-Castro, J.D.; Mantilla-Meluk, H.; Marmontel, M.; et al. Mercury in Populations of River Dolphins of the Amazon and Orinoco Basins. EcoHealth 2019, 16, 743–758. [Google Scholar] [CrossRef]
- Borges, Â.O.; Erickson, J.; Silva, L.A.D.; Fantin, C.; Domingos-Moreira, F.X.V. Mercury Bioaccumulation, Genotoxic and Biochemical Biomarkers Reveal the Health Status of Yellow-Spotted Amazon River Turtles (Podocnemis unifilis) in an Environmental Protection Area in the Amazon. Acta Amaz. 2022, 52, 254–263. [Google Scholar] [CrossRef]
- Pignati, M.; Pezzuti, J.; Souza, L.; Lima, M.; Pignati, W.; Mendes, R. Assessment of Mercury Concentration in Turtles (Podocnemis unifilis) in the Xingu River Basin, Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1185. [Google Scholar] [CrossRef]
- Targino, F.J.; Ribeiro, J.D.D.N.; Simões, J.S.; Carneiro, C.S.; Lazzarini, S.M.; Souza, A.R.; Ferreira, M.D.S.; Mano, S.B.; Mársico, E.T. Total Mercury Content in the Tissues of Freshwater Chelonium (Podocnemis expansa) and a Human Health Risk Assessment for the Amazon Population in Brazil. Int. J. Environ. Res. Public Health 2023, 20, 6489. [Google Scholar] [CrossRef]
- Oliveira, E.; Ignácio, A.R.A.; Lázaro, W.L.; Díez, S.; Guimarães, J.R.D.; Santos-Filho, M. Green Kingfishers as Sentinel Species for Mercury Contamination in Amazon. Arch. Environ. Contam. Toxicol. 2023, 85, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, T.C.; De Medeiros Costa, G.; De Carvalho, G.S.; Brum, B.R.; Ignácio, Á.R.A. Mercury and Methylmercury Concentration in the Feathers of Two Species of Kingfishers Megaceryle torquata and Chloroceryle amazona in the Upper Paraguay Basin and Amazon Basin. Ecotoxicology 2023, 32, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Galvão, R.C.F.; Holanda, I.B.B.; De Carvalho, D.P.; Almeida, R.; Souza, C.M.M.; Lacerda, L.D.; Bastos, W.R. Freshwater Shrimps (Macrobrachium depressimanum and Macrobrachium jelskii) as Biomonitors of Hg Availability in the Madeira River Basin, Western Amazon. Environ. Monit. Assess. 2018, 190, 77. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Ramírez-Mateos, V.; Da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; Do Nascimento, J.L.M.; et al. Large-Scale Projects in the Amazon and Human Exposure to Mercury: The Case-Study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Feitosa-Santana, C.; Souza, G.D.S.; Sirius, E.V.P.; Rodrigues, A.R.; Cortes, M.I.T.; Silveira, L.C.D.L.; Ventura, D.F. Color Vision Impairment with Low-Level Methylmercury Exposure of an Amazonian Population—Brazil. Neurotoxicology 2018, 66, 179–184. [Google Scholar] [CrossRef]
- Dos Santos Freitas, J.; Da Costa Brito Lacerda, E.M.; Da Silva Martins, I.C.V.; Rodrigues, D.; Bonci, D.M.O.; Cortes, M.I.T.; Corvelo, T.C.O.; Ventura, D.F.; De Lima Silveira, L.C.; Da Conceição Nascimento Pinheiro, M.; et al. Cross-Sectional Study to Assess the Association of Color Vision with Mercury Hair Concentration in Children from Brazilian Amazonian Riverine Communities. Neurotoxicology 2018, 65, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Costa Junior, J.M.F.; Lima, A.A.D.S.; Rodrigues Junior, D.; Khoury, E.D.T.; Souza, G.D.S.; Silveira, L.C.D.L.; Pinheiro, M.D.C.N. Manifestações Emocionais e Motoras de Ribeirinhos Expostos Ao Mercúrio Na Amazônia. Rev. Bras. Epidemiol. 2017, 20, 212–224. [Google Scholar] [CrossRef]
- Oliveira, R.A.A.D.; Pinto, B.D.; Rebouças, B.H.; Ciampi De Andrade, D.; Vasconcellos, A.C.S.D.; Basta, P.C. Neurological Impacts of Chronic Methylmercury Exposure in Munduruku Indigenous Adults: Somatosensory, Motor, and Cognitive Abnormalities. Int. J. Environ. Res. Public Health 2021, 18, 10270. [Google Scholar] [CrossRef]
- Perini, J.A.; Silva, M.C.; Vasconcellos, A.C.S.D.; Viana, P.V.S.; Lima, M.O.; Jesus, I.M.; Kempton, J.W.; Oliveira, R.A.A.; Hacon, S.S.; Basta, P.C. Genetic Polymorphism of Delta Aminolevulinic Acid Dehydratase (ALAD) Gene and Symptoms of Chronic Mercury Exposure in Munduruku Indigenous Children within the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 8746. [Google Scholar] [CrossRef]
- Vianna, A.D.S.; Câmara, V.D.M.; Barbosa, M.C.D.M.; Santos, A.D.S.E.; Asmus, C.I.R.F.; Luiz, R.R.; Jesus, I.M.D. Exposição Ao Mercúrio e Anemia Em Crianças e Adolescentes de Seis Comunidades Da Amazônia Brasileira. Ciênc. Saúde Coletiva 2022, 27, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.K.; Chhabria, R.; Hafzalla, G.W.; Giffin, L.; Kucharski, K.; Myers, K.; Culquichicón, C.; Montero, S.; Lescano, A.G.; Vega, C.M.; et al. Impairment in Working Memory and Executive Function Associated with Mercury Exposure in Indigenous Populations in Upper Amazonian Peru. Int. J. Environ. Res. Public Health 2022, 19, 10989. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.; Ortiz, E.; Feingold, B.; Berky, A.; Diringer, S.; Morales, A.; Jurado, E.; Hsu-Kim, H.; Pan, W. Spatial, Temporal, and Dietary Variables Associated with Elevated Mercury Exposure in Peruvian Riverine Communities Upstream and Downstream of Artisanal and Small-Scale Gold Mining. Int. J. Environ. Res. Public Health 2017, 14, 1582. [Google Scholar] [CrossRef]
- Weinhouse, C.; Ortiz, E.J.; Berky, A.J.; Bullins, P.; Hare-Grogg, J.; Rogers, L.; Morales, A.-M.; Hsu-Kim, H.; Pan, W.K. Hair Mercury Level Is Associated with Anemia and Micronutrient Status in Children Living Near Artisanal and Small-Scale Gold Mining in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2017, 97, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Koenigsmark, F.; Weinhouse, C.; Berky, A.; Morales, A.; Ortiz, E.; Pierce, E.; Pan, W.; Hsu-Kim, H. Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2021, 18, 13350. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, A.C.S.D.; Barrocas, P.R.G.; Ruiz, C.M.V.; Mourão, D.D.S.; Hacon, S.D.S. Burden of Mild Mental Retardation Attributed to Prenatal Methylmercury Exposure in Amazon: Local and Regional Estimates. Ciênc. Saúde Coletiva 2018, 23, 3535–3545. [Google Scholar] [CrossRef]
- De Paula Fonseca Arrifano, G.; Del Carmen Rodriguez Martin-Doimeadios, R.; Jiménez-Moreno, M.; Augusto-Oliveira, M.; Rogério Souza-Monteiro, J.; Paraense, R.; Rodrigues Machado, C.; Farina, M.; Macchi, B.; Do Nascimento, J.L.M.; et al. Assessing Mercury Intoxication in Isolated/Remote Populations: Increased S100B mRNA in Blood in Exposed Riverine Inhabitants of the Amazon. NeuroToxicology 2018, 68, 151–158. [Google Scholar] [CrossRef]
- Cerbino, M.R.; Vieira, J.C.S.; Braga, C.P.; Oliveira, G.; Padilha, I.F.; Silva, T.M.; Zara, L.F.; Silva, N.J.; Padilha, P.M. Metalloproteomics Approach to Analyze Mercury in Breast Milk and Hair Samples of Lactating Women in Communities of the Amazon Basin, Brazil. Biol. Trace Elem. Res. 2018, 181, 216–226. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Fernández-Trujillo, S.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Alvarez-Leite, J.I.; Do Nascimento, J.L.M.; Amador, M.T.; et al. Genetic Susceptibility to Neurodegeneration in Amazon: Apolipoprotein E Genotyping in Vulnerable Populations Exposed to Mercury. Front. Genet. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.J.X.; Arain, A.; Fernandez, L.E. Mercury Exposure, Risk Factors, and Perceptions among Women of Childbearing Age in an Artisanal Gold Mining Region of the Peruvian Amazon. Environ. Res. 2019, 179, 108786. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.S.; Lacerda, E.M.C.B.; Rodrigues Júnior, D.; Corvelo, T.C.O.; Silveira, L.C.L.; Pinheiro, M.D.C.N.; Souza, G.S. Mercury Exposure of Children Living in Amazonian Villages: Influence of Geographical Location Where They Lived during Prenatal and Postnatal Development. An. Acad. Bras. Ciênc. 2019, 91, e20180097. [Google Scholar] [CrossRef]
- Santos-Lima, C.D.; Mourão, D.D.S.; Carvalho, C.F.D.; Souza-Marques, B.; Vega, C.M.; Gonçalves, R.A.; Argollo, N.; Menezes-Filho, J.A.; Abreu, N.; Hacon, S.D.S. Neuropsychological Effects of Mercury Exposure in Children and Adolescents of the Amazon Region, Brazil. Neurotoxicology 2020, 79, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Frischtak, H.; Berky, A.; Ortiz, E.J.; Morales, A.M.; Hsu-Kim, H.; Pendergast, L.L.; Pan, W.K. Elevated Hair Mercury Levels Are Associated with Neurodevelopmental Deficits in Children Living Near Artisanal and Small-Scale Gold Mining in Peru. GeoHealth 2020, 4, e2019GH000222. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.C.; Hurtado-Gonzales, J.L.; Chin, C.J.; Persaud, J. Survey of Methylmercury Exposures and Risk Factors among Indigenous Communities in Guyana, South America. J. Health Pollut. 2020, 10, 200604. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, E.M.D.C.B.; Souza, G.D.S.; Cortes, M.I.T.; Rodrigues, A.R.; Pinheiro, M.C.N.; Silveira, L.C.D.L.; Ventura, D.F. Comparison of Visual Functions of Two Amazonian Populations: Possible Consequences of Different Mercury Exposure. Front. Neurosci. 2020, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, C.; Gallis, J.A.; Ortiz, E.; Berky, A.J.; Morales, A.M.; Diringer, S.E.; Harrington, J.; Bullins, P.; Rogers, L.; Hare-Grogg, J.; et al. A Population-Based Mercury Exposure Assessment near an Artisanal and Small-Scale Gold Mining Site in the Peruvian Amazon. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bello, T.C.S.; Buralli, R.J.; Cunha, M.P.L.; Dórea, J.G.; Diaz-Quijano, F.A.; Guimarães, J.R.D.; Marques, R.C. Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil. Int. J. Environ. Res. Public Health 2023, 20, 5225. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Araújo, A.; Arrifano, G.P.; Macchi, B.M.; Augusto-Oliveira, M.; Santos-Sacramento, L.; Rodríguez Martín-Doimeadios, R.C.; Jiménez-Moreno, M.; Martins Filho, A.J.; Alvarez-Leite, J.I.; Oriá, R.B.; et al. Hair Mercury Is Associated with Dyslipidemia and Cardiovascular Risk: An Anthropometric, Biochemical and Genetic Cross-Sectional Study of Amazonian Vulnerable Populations. Environ. Res. 2023, 229, 115971. [Google Scholar] [CrossRef]
- Suárez-Criado, L.; Rodríguez-González, P.; Marrugo-Negrete, J.; García Alonso, J.I.; Díez, S. Determination of Methylmercury and Inorganic Mercury in Human Hair Samples of Individuals from Colombian Gold Mining Regions by Double Spiking Isotope Dilution and GC-ICP-MS. Environ. Res. 2023, 231, 115970. [Google Scholar] [CrossRef]
- Silva, M.C.D.; Oliveira, R.A.A.D.; Vasconcellos, A.C.S.D.; Rebouças, B.H.; Pinto, B.D.; Lima, M.D.O.; Jesus, I.M.D.; Machado, D.E.; Hacon, S.S.; Basta, P.C.; et al. Chronic Mercury Exposure and GSTP1 Polymorphism in Munduruku Indigenous from Brazilian Amazon. Toxics 2023, 11, 138. [Google Scholar] [CrossRef]
- Pignoux, R.; Gourves, P.-Y.; Sow, M.; Maury-Brachet, R. Imprégnation mercurielle des femmes enceintes de Guyane (Haut Maroni): Étude et prévention. Toxicol. Anal. Clin. 2019, 31, 37–48. [Google Scholar] [CrossRef]
- Cunha, M.; Marques, R.; Dórea, J. Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon. Nutrients 2018, 10, 1146. [Google Scholar] [CrossRef]
- Bastos, W.R.; Vieira, S.M.; Manzatto, Â.G.; Dórea, J.G.; Rubira, M.C.; De Souza, V.F.P.; Da Costa Junior, W.A.; Souza Bastos, M.T. Heterogeneity of Multimedia Exposures to Neurotoxic Elements (Al, As, Cd, Pb, Mn, and Hg) in Breastfed Infants from Porto Velho, Brazil. Biol. Trace Elem. Res. 2018, 184, 7–15. [Google Scholar] [CrossRef]
- Carvalho, L.V.B.; Hacon, S.S.; Vega, C.M.; Vieira, J.A.; Larentis, A.L.; Mattos, R.C.O.C.; Valente, D.; Costa-Amaral, I.C.; Mourão, D.S.; Silva, G.P.; et al. Oxidative Stress Levels Induced by Mercury Exposure in Amazon Juvenile Populations in Brazil. Int. J. Environ. Res. Public Health 2019, 16, 2682. [Google Scholar] [CrossRef]
- Oliveira, A.T.D.; Rodrigues, P.A.; Ramos Filho, A.M.; Gomes, M.F.S.; Liebl, A.R.S.; de Pinho, J.V.; Aride, P.H.R.; Conte-Junior, C.A. Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon. Int. J. Environ. Res. Public Health 2023, 20, 6990. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Umesh, M.; Shanmugam, M.K.; Chakraborty, P.; Duhan, L.; Gummadi, S.N.; Pasrija, R.; Jayaraj, I.; Dasarahally Huligowda, L.K. A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects. Sustainability 2023, 15, 13292. [Google Scholar] [CrossRef]
- Casagrande, G.C.R.; Dambros, J.; de Andrade, E.A.; Martello, F.; Sobral-Souza, T.; Moreno, M.I.C.; Battirola, L.D.; de Andrade, R.L.T. Atmospheric Mercury in Forests: Accumulation Analysis in a Gold Mining Area in the Southern Amazon, Brazil. Environ. Monit. Assess. 2023, 195, 477. [Google Scholar] [CrossRef] [PubMed]
- Esteban-López, M.; Arrebola, J.P.; Juliá, M.; Pärt, P.; Soto, E.; Cañas, A.; Pedraza-Díaz, S.; González-Rubio, J.; Castaño, A. Selecting the Best Non-Invasive Matrix to Measure Mercury Exposure in Human Biomonitoring Surveys. Environ. Res. 2022, 204, 112394. [Google Scholar] [CrossRef] [PubMed]
- Santos Serrão De Castro, N.; De Oliveira Lima, M. Hair as a Biomarker of Long Term Mercury Exposure in Brazilian Amazon: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 500. [Google Scholar] [CrossRef]
- Pestana, I.A.; Almeida, M.G.; Bastos, W.R.; Souza, C.M.M. Total Hg and Methylmercury Dynamics in a River-Floodplain System in the Western Amazon: Influence of Seasonality, Organic Matter and Physical and Chemical Parameters. Sci. Total Environ. 2019, 656, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Verschoor, G. Re-Imagining Environmental Governance: Gold Dredge Mining vs Territorial Health in the Colombian Amazon. Geoforum 2020, 117, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, J.; Gasparinetti, P.; Bakker, L.B.; Lobo, F.; Nagel, G. Socioeconomic Cost of Dredge Boat Gold Mining in the Tapajós Basin, Eastern Amazon. Resour. Policy 2022, 79, 103102. [Google Scholar] [CrossRef]
- Junk, W.J.; Wittmann, F.; Schöngart, J.; Piedade, M.T.F. A Classification of the Major Habitats of Amazonian Black-Water River Floodplains and a Comparison with Their White-Water Counterparts. Wetl. Ecol. Manag. 2015, 23, 677–693. [Google Scholar] [CrossRef]











| Arguments | Search Syntax |
|---|---|
| mercury in the Amazon | Amazon * AND (mercury * OR hg OR MeHg OR methyl * OR “total mercury”) |
| activity | AND (mining OR artisanal OR asgm OR “gold mining *” OR garimp * OR alluvial OR contamination OR “inorganic contaminant *” OR “trace mineral *” OR trophic) |
| specimen | AND (fish OR species OR population OR aquatic OR bioaccumulation OR community OR riverine OR “human hair” OR indigenous OR human OR nonhuman OR animal) |
| conditions | AND (exposure OR accumulation OR concentration OR content OR consumption) |
| biomarker | AND (tissue OR muscle OR protein OR hair OR blood) |
| exclude ex situ | NOT (farm OR agriculture) |
| Hydrographic Basin * | Mentioned Mining? | Country | Study | Species/Feeding Guild | THg (μg/g) Based on Mean/Median † |
|---|---|---|---|---|---|
| A | No | Brazil | [64,65] ** | Hoplias malabaricus, carnivore | 0.947 † |
| A | No | Brazil | [66] | Carcharhinus acronotus, carnivore | 1.120 |
| A, T | Yes | Brazil | [67] | Arapaima sp., carnivore | 0.375 |
| A, T | No | Brazil | [68] | Plagioscion squamosisYesus, carnivore | 1.510 |
| A | Yes | Brazil | [69] | Pseudoplatystoma tigrinum, carnivore | 0.920 |
| A | No | Brazil | [70] | Ageneiosus inermis, carnivore | 0.691 |
| A | No | Brazil | [71] | Acestrorhyncus falcirostris, carnivore | 1.490 |
| A | No | Brazil | [72] | Colomesus asellus, omnivore | 0.350 |
| T | Yes | Brazil | [73] | Carnivorous group: Cichla monoculus, Plagioscion squamosis Yesus, and Serrasalmus calmoni | 0.668 |
| T | Yes | Brazil | [74] | Plagioscion squamosis Yesus, carnivore | 0.730 |
| T | Yes | Brazil | [75] | Cichla pinima, carnivore | 1.172 |
| T | No | Brazil | [76] | Serrasalmus rhombeus, carnivore | 0.088 |
| M | No | Brazil | [77] | Carnivorous group: Plasgioscion squamosis Yesus, Calophysus macropterus, Cichla pleiozona, and Hoplias malabaricus | 0.970 |
| M | No | Brazil | [78] | Prochilodus nigricans, detritivore | 0.064 |
| M | No | Brazil | [79] | Serrasalmus rhombeus, carnivore | 0.263 |
| M | Yes | Brazil | [80] | Triportheus angulatus, omnivore | 0.290 |
| M | No | Brazil | [81] | Triportheus albus, omnivore | 0.029 |
| M | No | Brazil | [82] | Pinirampus pirinampu, benthivore | 0.060 |
| M | No | Brazil | [83] | Arapaima gigas, carnivore | 0.153 |
| M | No | Brazil | [84] | Serrasalmus rhombeus, carnivore | 1.640 |
| M | No | Brazil | [85] | Plagioscion squamosisYesus, carnivore and Colossoma macropomum, omnivore | 0.086 |
| M | No | Brazil | [86] | Brachyplatystoma filamentosum, carnivore | 0.402 |
| M | No | Brazil | [87] | Calophysus maropterus, carnivore | 1.400 |
| M | No | Peru/Brazil (Border) | [88] | Calophysus maropterus, carnivore | 0.229 |
| M | No | Brazil | [89] | Cichla spp., carnivore | 0.128 |
| M | No | Brazil | [90] | Semaprochilodus spp. (Jaraqui), detritivore | 0.132 |
| M | No | Brazil | [91] | Serrasalmus rhombeus, carnivore | 0.268 |
| M | No | Brazil | [92] | Cichla spp. (Tucunaré), carnivore | 0.435 |
| M | Yes | Brazil | [93] | Serrasalmus rhombeus, carnivore | 0.417 |
| X | No | Brazil | [94] | Hemiodus unimaculatus, omnivore | 0.480 |
| Tp | No | Brazil | [95] | Serrasalmus rhombeus, carnivore | 0.304 |
| Tp | No | Brazil | [96] | Brycon falcatus, omnivore | 0.052 |
| Ar | Yes | Brazil | [12] | Carnivorous group: Ageneiosus inermis, Boulengerella cuvieri, Cichla monoculus, and Hoplias aimara | 0.580 |
| Ar | Yes | Brazil | [97] | Curimata incompta, detritivore | 0.370 |
| Ar | Yes | Brazil | [98] | Plagioscion squamosis Yesus, carnivore | 0.320 † |
| J | No | Brazil | [99] | Plagioscion squamosis Yesus, carnivore | 1.090 |
| MD | Yes | Peru | [100] | Serrasalmus spp., carnivore | 0.280 |
| MD | Yes | Peru | [101] | Serrasalmus spp., carnivore | 3.720 |
| B | No | Bolivia | [102] | Brycon amazonicus, omnivore | 0.700 |
| Br | Yes | Brazil | [103] | Pinirampus pirinampu, carnivore | 0.869 |
| Br | Yes | Brazil | [32] | Pygocentrus nattereri, carnivore | 1.215 |
| M | Yes | Brazil | [104] | Serrasalmus rhombeus, carnivore | 0.283 |
| Su | Yes | Suriname | [105] | Multiple, high THg in carnivores: Acestrorhynchus microlepsis, Hoplias malabaricus, Cichla ocellaris, Serrasalmus rhombeus, and Pristobrycon eigenmanni | 2.528 |
| C | Yes | French Guiana | [106] | Hoplias aimara and Boulengerella cuvieri, carnivore | 2.900 |
| Mz | Yes | Guyana | [107] | Ageneiosus ucayalensis, carnivore | 5.920 |
| Location | Mentioned Mining? | Study | Species | Tissue | Mean THg (μg/g) |
|---|---|---|---|---|---|
| Madeira River, Rondônia, Brazil | No | [108] | Arapaima gigas, fish, carnivore | hepatic | 17.420 |
| Araguari River, Amapá, Brazil | No | [109] | Anodus orinocensis, fish, omnivore | hepatic | 0.500 |
| Madeira River, Brazil | No | [110] | Macrobrachium amazonicum, shrimp, omnivore | muscle | 0.610 |
| Atlantic Coast, Ilha dos Caranguejos, Brazil | No | [111] | Sciades herzbergii, fish, omnivore | muscle | 0.033 |
| Mamirauá, Amazonas, Brazil | No | [112] | Panthera onca, mammal, carnivore | pelage | 17.900 |
| Guaporé River, Brazil | No | [113] | Inia boliviensis, mammal, piscivore | adipose | 1.323 |
| Beni River, Bolívia | No | [114] | Caiman yacare, reptile, piscivore | muscle | 0.150 |
| Madeira River, Brazil | No | [115] | Ardea cocoi, bird, carnivore | feather | 4.046 |
| Biological Station Cocha Cashu, Peru | No | [116] | Rhynconycteris naso, mammal, carnivore | pelage | 7.440 |
| Madre de Dios, Peru | Yes | [117] | Phyllostomus elongatus, mammal, carnivore | pelage | 0.660 |
| Figueiredo, Amazonas, Brazil | No | [118] | Trichechus inunguis, mammal, herbivore | muscle | 0.059 |
| Arauca River and Orinoco River, Colombia | Yes | [119] | Inia sp. and Sotalia sp., mammal, piscivore | muscle | 0.870 |
| Itapuru mirim Lagoon, Brazil | No | [120] | Podocnemis unifilis, reptile, herbivore | muscle | 0.011 |
| Xingu and Teles Pires’ Rivers, Brazil | No | [121] | Podocnemis unifilis, reptile, herbivore | muscle | 0.134 |
| Uatumã River, Balbina Brazil | No | [122] | Podocnemis expansa, reptile, omnivore | muscle | 0.109 |
| Teles Pires, Brazil | No | [123] | Chloroceryle amazona, bird, piscivore | feather | 11.570 |
| Teles Pires, Brazil | No | [124] | Chloroceryle amazona, bird, piscivore | feather | 4.000 |
| Madeira River, Brazil | No | [125] | Macrobrachium depres Yesanum, Macrobrachium jelskii, shrimp, omnivore | muscle | 0.022 |
| Hydrographic Basin * | Mining Mentioned? | Country | Study | Community/Population | THg (μg/g) Based on Mean/Median † |
|---|---|---|---|---|---|
| To | Yes | Brazil | [126] | Adults (18–70), fish as a staple food, near the reservoir of Tucuruí Dam. | 10.900 |
| U | Yes | Brazil | [8] | Age groups and comparison of various villages. Yanomami indigenous reserve, with a high diet of fish and nearby mining activity. | 15.500 † |
| M | Yes | Brazil | [127] | Age groups of adults ranging from 17 to 92 years. | 26.030 |
| To, Ta | Yes | Brazil | [128] | Adult riverside dwellers only (18 to 60 years old). | 4.500 |
| Ta | Yes | Brazil | [129] | Riverside dwellers only; adult women (13 to 53 years old). | 9.150 |
| Ta | Yes | Brazil | [130] | Munduruku Indigenous Reserve. Comparison between villages. | 7.400 |
| M, Ta | No | Brazil | [74] | Pregnant women (18 to 40 years old). Fish Diet. | 6.070 |
| MD | Yes | Peru | [62] | Urban and rural demographic comparison with a focus on fish diets. | 1.740 |
| A | Yes | Colombia | [69] | Indigenous community in Puerto Nariño. Mean age~35 years. Diet rich in fish. | 5.310 |
| Ta | No | Brazil | [11] | Munduruku Indigenous Reserve. Comparison between villages and age categorization with juvenile, childbearing-age, and other adults. | 11.500 |
| Ta | No | Brazil | [131] | Munduruku Indigenous Reserve. Comparison between villages and the exclusively juvenile population. | 11.800 |
| Ta | No | Brazil | [10] | Munduruku Indigenous Reserve. Comparison between villages. Ages > 12 years. | 7.400 |
| A, Cg, Ta | Yes | Brazil | [132] | Youth and adults in riverside communities. | 12.700 |
| MD | Yes | Peru | [133] | Matsigenka Indigenous community (ages 1 to 65). | 11.830 |
| MD | No | Peru | [134] | Riverside communities. High-fish diet. | 4.800 |
| MD | No | Peru | [135] | Comparison of various dwellings in the Amarakaeri Reserve, age categorization (under 5 years old, and 5 to 11 years old). | 1.030 † |
| MD | Yes | Peru | [136] | Comparison of various dwellings in the Amarakaeri Reserve. | 4.150 |
| A | Yes | Brazil | [137] | Riverside population. Prenatal exposure, women of childbearing age (15 to 49 years) | 6.490 |
| To, Ta | No | Brazil | [138] | Riverside communities with ages between 19 and 70 years (high THg). | 15.900 † |
| M, N | Yes | Brazil | [139] | Lactating women. | 2.120 |
| To | No | Brazil | [140] | Riverine populations in the Tucuruí Dam reservoir area. | 8.120 † |
| CP | Yes | Colombia | [26] | Indigenous communities in Tarapacá village. | 17.800 † |
| MD | Yes | Peru | [141] | Women of childbearing age. | 5.500 |
| Ta, To | No | Brazil | [142] | Children from riverside villages; born to women aged between 25 and 40. Fish-rich diet and primary exposure to Hg. | 22.380 |
| M | No | Brazil | [143] | Children/adolescents aged 6 to 14 along the Madeira River. | 3.070 |
| MD | Yes | Peru | [144] | Comparison of various dwellings in the Amarakaeri Reserve. Indigenous Native children (6 to 15 years old) | 2.060 |
| RK | Yes | Guyana | [145] | Indigenous people from the Rupununi region (15 to 78 years old). High-fish diet. Comparison of mining area and control area. | 27.620 |
| Ta, To | Yes | Brazil | [146] | Riverine men and miners and the THg among them. High-fish diet. Itaituba and Serra Pelada. | 20.000 |
| MD | Yes | Peru | [147] | Comparison of various dwellings in the Amarakaeri Reserve. Women of childbearing age (15 to 49 years). | 3.500 † |
| M | Yes | Brazil | [148] | Riverine, rural, mining, and urban communities. Women of childbearing age. | 12.220 † |
| To | No | Brazil | [149] | Adults (18–70 years), Riverine populations in the Tucuruí Dam reservoir area. | 7.900 † |
| Ap | Yes | Colombia | [150] | Population in different locations in mining regions. High-fish diet. | 14.920 |
| Ta | No | Brazil | [151] | Munduruku indigenous reserve. Comparison between villages and categorization. Diet rich in fish. Ages over 12 years old. | 8.500 |
| Ap | Yes | Colombia | [14] | Indigenous population of the Yaigojé Apaporis National Natural Park. | 34.900 |
| Ta | No | Brazil | [53] | Adult riverside residents only (18 to 60 years old). High consumption of fish. | 10.800 |
| Co | Yes | French Guiana | [152] | Only pregnant women, ethnic groups considered (15 to 41 years old); tribal and indigenous communities of Wayana. | 12.800 |
| M | No | Brazil | [153] | Mothers and children in childbirth and after pregnancy at 6, 24, and 59 months of age. | 11.610 |
| GLM | Logistic Regression | ||
|---|---|---|---|
| AIC | 257.26 | 53.867 | |
| Parameters | p-Value | p-Value | |
| THg | Children | 0.6055 | 0.416 |
| Indigenous | 0.4201 | 0.273 | |
| Maternity | 0.2364 | 0.661 | |
| Riverside | 0.6108 | 0.287 | |
| Mining | 0.2413 | 0.774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martoredjo, I.; Calvão Santos, L.B.; Vilhena, J.C.E.; Rodrigues, A.B.L.; de Almeida, A.; Sousa Passos, C.J.; Florentino, A.C. Trends in Mercury Contamination Distribution among Human and Animal Populations in the Amazon Region. Toxics 2024, 12, 204. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics12030204
Martoredjo I, Calvão Santos LB, Vilhena JCE, Rodrigues ABL, de Almeida A, Sousa Passos CJ, Florentino AC. Trends in Mercury Contamination Distribution among Human and Animal Populations in the Amazon Region. Toxics. 2024; 12(3):204. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics12030204
Chicago/Turabian StyleMartoredjo, Irvin, Lenize Batista Calvão Santos, Jéssica Caroline Evangelista Vilhena, Alex Bruno Lobato Rodrigues, Andréia de Almeida, Carlos José Sousa Passos, and Alexandro Cezar Florentino. 2024. "Trends in Mercury Contamination Distribution among Human and Animal Populations in the Amazon Region" Toxics 12, no. 3: 204. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics12030204