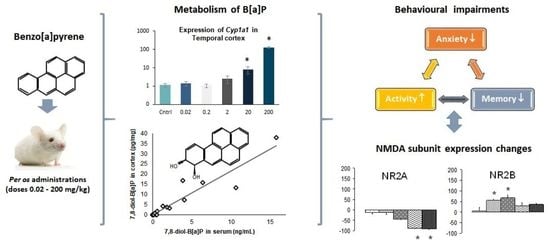
Assessment of 9-OH- and 7,8-diol-benzo[a]pyrene in Blood as Potent Markers of Cognitive Impairment Related to benzo[a]pyrene Exposure: An Animal Model Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Serum and Brain Concentration of B[a]P and Hydroxylated B[a]P Metabolites
2.3. Nmda and Cytochrome Gene Expression
2.4. Behavioural Testing
2.5. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Concentration Levels of B[a]P and Hydroxylated-Metabolites in Serum and Brain
3.3. Expression of Cyp1a1 and Cyp1b1
3.4. Expression of Nmda Subunits
3.5. Behavioural Effects of B[a}P
3.5.1. Elevated Plus Maze
3.5.2. Hole Board Apparatus
3.5.3. Y Maze
3.5.4. Morris Water Maze
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, A.; Ji, G.; Jiang, T.; Lu, A.; You, Y.; Liu, N.; Luo, C.; Yan, W.; Zhao, P. Contributions of aryl hydrocarbon receptor genetic variants to the risk of glioma and PAH-DNA adducts. Toxicol. Sci. 2012, 128, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrington-Trimis, J.L.; Searles Nielsen, S.; Preston-Martin, S.; Gauderman, W.J.; Holly, E.A.; Farin, F.M.; Mueller, B.A.; McKean-Cowdin, R. Parental smoking and risk of childhood brain tumors by functional polymorphisms in polycyclic aromatic hydrocarbon metabolism genes. PLoS ONE 2013, 8, e79110. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pang, S.; Zhang, X.; Li, X.; Sun, Y.; Lu, X.; Zhang, Q.; Zhang, Z. Levels and neurodevelopmental effects of polycyclic aromatic hydrocarbons in settled house dust of urban dwellings on preschool–aged children in Nanjing, China. Atmos. Pollut. Res. 2014, 5, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.S.; Rauh, V.A.; Bansal, R.; Hao, X.; Toth, Z.; Nati, G.; Walsh, K.; Miller, R.L.; Arias, F.; Semanek, D.; et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the develop ent of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 2015, 72, 531–540. [Google Scholar] [CrossRef]
- Grova, N.; Valley, A.; Turner, J.D.; Morel, A.; Muller, C.P.; Schroeder, H. Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. Neurotoxicology 2007, 28, 630–636. [Google Scholar] [CrossRef]
- Grova, N.; Schroeder, H.; Farinelle, S.; Prodhomme, E.; Valley, A.; Muller, C.P. Sub-acute administration of benzo[a]pyrene (B[a]P) reduces anxiety-related behaviour in adult mice and modulates regional expression of N-methyl-D-aspartate (NMDA) receptors genes in relevant brain regions. Chemosphere 2008, 73, S295–S302. [Google Scholar] [CrossRef]
- Bouayed, J.; Desor, F.; Rammal, H.; Kiemer, A.K.; Tybl, E.; Schroeder, H.; Rychen, G.; Soulimani, R. Effects of lactational exposure to benzo[a]pyrene (B[a]P) on postnatal neurodevelopment, neuronal receptor gene expression and behaviour in mice. Toxicology 2009, 259, 97–106. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Nie, J.-S.; Wang, F.; Shi, Y.-T.; Zhang, L.; Antonucci, A.; Liu, H.-J.; Wang, J.; Zhao, J.; Zhang, Q.-L.; et al. Effects of benzo[a]pyrene on autonomic nervous system of coke oven workers. J. Occup. Health 2008, 50, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Mahbub, R.; Fox, J.G. Xenobiotics: Interaction with the Intestinal Microflora. ILAR J. 2015, 56, 218–227. [Google Scholar] [CrossRef]
- Chepelev, N.L.; Moffat, I.D.; Bowers, W.J.; Yauk, C.L. Neurotoxicity may be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment. Mutat. Res. Rev. Mutat. Res. 2015, 764, 64–89. [Google Scholar] [CrossRef]
- Majchrzak, R.; Sroczyński, J.; Chełmecka, E. Evaluation of the nervous system in workers in the furnace and coal divisions of the coke-producing plants. Med. Pr. 1990, 41, 108–113. [Google Scholar] [PubMed]
- Niu, Q.; Zhang, H.; Li, X.; Li, M. Benzo[a]pyrene-induced neurobehavioral function and neurotransmitter alterations in coke oven workers. Occup. Environ. Med. 2010, 67, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Peng, B.; Cheng, S.; Xia, Y.; Tu, B. The effect of occupational exposure to benzo[a]pyrene on neurobehavioral function in coke oven workers. Am. J. Ind. Med. 2013, 56, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, K.H.; Warshaw, R.H. Neurotoxic effects from residential exposure to chemicals from an oil reprocessing facility and superfund site. Neurotoxicol. Teratol. 1995, 17, 89–102. [Google Scholar] [CrossRef]
- Rhomberg, L.R.; Bowers, T.S.; Beyer, L.A.; Goodman, J.E. Comment on “Residential and biological exposure assessment of chemicals from a wood treatment plant” by James Dahlgren et al. [Chemosphere 67(9) (2007) S279-S285]. Chemosphere 2008, 70, 1730–1733; author reply 1734–1736. [Google Scholar] [CrossRef]
- Das, L.; Patel, B.; Patri, M. Adolescence benzo[a]pyrene treatment induces learning and memory impairment and anxiolytic like behavioral response altering neuronal morphology of hippocampus in adult male Wistar rats. Toxicol. Rep. 2019, 6, 1104–1113. [Google Scholar] [CrossRef]
- Grova, N.; Salquèbre, G.; Schroeder, H.; Appenzeller, B.M.R. Determination of PAHs and OH- PAHs in rat brain by gas chromatography tandem (triple quadrupole) mass spectrometry. Chem. Res. Toxicol. 2011, 24, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Chahin, A.; Peiffer, J.; Olry, J.-C.; Crepeaux, G.; Schroeder, H.; Rychen, G.; Guiavarc’h, Y. EROD activity induction in peripheral blood lymphocytes, liver and brain tissues of rats orally exposed to poly cyclic aromatic hydrocarbons. Food Chem. Toxicol. 2013, 56, 371–380. [Google Scholar] [CrossRef]
- Crépeaux, G.; Grova, N.; Bouillaud-Kremarik, P.; Sikhayeva, N.; Salquèbre, G.; Rychen, G.; Soulimani, R.; Appenzeller, B.; Schroeder, H. Short-term effects of a perinatal exposure to a 16 polycyclic aromatic hydrocarbon mixture in rats: Assessment of early motor and sensorial development and cerebral cytochrome oxidase activity in pups. Neurotoxicology 2014, 43, 90–101. [Google Scholar] [CrossRef]
- Saunders, C.R.; Das, S.K.; Ramesh, A.; Shockley, D.C.; Mukherjee, S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: Role of oxidative stress. J. Appl. Toxicol. 2006, 26, 427–438. [Google Scholar] [CrossRef]
- Tajima, N.; Karakas, E.; Grant, T.; Simorowski, N.; Diaz-Avalos, R.; Grigorieff, N.; Furukawa, H. Activation of NMDA receptors and the mechanism of inhibition by ifenprodil. Nature 2016, 534, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Kakeyama, M. Development of Higher Brain Function Tests in Rodents and Its Application to Neurotoxicity Assessment of Environmental Chemicals. Nihon Eiseigaku Zasshi 2015, 70, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, F.; Zheng, J.; Li, S.; Qiang, M. Chronic Administration of Benzo(a)pyrene Induces Memory Impairment and Anxiety-Like Behavior and Increases of NR2B DNA Methylation. PLoS ONE 2016, 11, e0149574. [Google Scholar] [CrossRef] [PubMed]
- Patri, M.; Singh, A.; Mallick, B.N. Protective role of noradrenaline in benzo[a]pyrene-induced learning impairment in developing rat. J. Neurosci. Res. 2013, 91, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, J.; Cosnier, F.; Grova, N.; Nunge, H.; Salquèbre, G.; Decret, M.-J.; Cossec, B.; Rychen, G.; Appenzeller, B.M.R.; Schroeder, H. Neurobehavioral toxicity of a repeated exposure (14 days) to the airborne polycyclic aromatic hydrocarbon fluorene in adult Wistar male rats. PLoS ONE 2013, 8, e71413. [Google Scholar] [CrossRef] [Green Version]
- Saunders, C.R.; Shockley, D.C.; Knuckles, M.E. Fluoranthene-induced neurobehavioral toxicity In F-344 rats. Int. J. Toxicol. 2003, 22, 263–276. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Xia, E.-Q.; Xu, X.-R.; Li, S.; Ling, W.-H.; Wu, S.; Deng, G.-F.; Zou, Z.-F.; Zhou, J.; Li, H.-B. Evaluation of Benzo[a]pyrene in Food from China by High-Performance Liquid Chromatography-Fluorescence Detection. Int. J. Environ. Res. Public Health 2012, 9, 4159–4169. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives & World Health Organization. (2006). Evaluation of certain food contaminants: Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/43258 (accessed on 7 April 2020).
- Fox, J.; Spassova, M.; Flowers, L. Toxicological Review Benzo[a]Pyrene. National Center for Environmental Assessment (Washington). 2017. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0136tr.pdf (accessed on 7 April 2020).
- Schellenberger, M.T.; Grova, N.; Willième, S.; Farinelle, S.; Prodhomme, E.J.F.; Muller, C.P. Modulation of benzo[a]pyrene induced immunotoxicity in mice actively immunized with a B[a]P-diphtheria toxoid conjugate. Toxicol. Appl. Pharmacol. 2009, 240, 37–45. [Google Scholar] [CrossRef]
- Schote, A.B.; Turner, J.D.; Schiltz, J.; Muller, C.P. Nuclear receptors in human immune cells: Expression and correlations. Mol. Immunol. 2007, 44, 1436–1445. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Brown, G.R.; Nemes, C. The exploratory behaviour of rats in the hole-board apparatus: Is head-dipping a valid measure of neophilia? Behav. Process. 2008, 78, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, M.C.; Sandner, G.; Graeff, F.G. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav. Brain Res. 1993, 56, 115–118. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorhees, C.V.; Williams, M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, R.J.; Johnson, N.J. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 1995, 52, 297–303. [Google Scholar] [CrossRef]
- do-Rego, J.-C.; Viana, A.F.; Le Maître, E.; Deniel, A.; Rates, S.M.K.; Leroux-Nicollet, I.; Costentin, J. Comparisons between anxiety tests for selection of anxious and non anxious mice. Behav. Brain Res. 2006, 169, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef]
- Granberg, L.; Ostergren, A.; Brandt, I.; Brittebo, E.B. CYP1A1 and CYP1B1 in blood-brain interfaces: CYP1A1-dependent bioactivation of 7,12-dimethylbenz(a)anthracene in endothelial cells. Drug Metab. Dispos. 2003, 31, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Violle, N.; Balandras, F.; Le Roux, Y.; Desor, D.; Schroeder, H. Variations in illumination, closed wall transparency and/or extramaze space influence both baseline anxiety and response to diazepam in the rat elevated plus-maze. Behav. Brain Res. 2009, 203, 35–42. [Google Scholar] [CrossRef]
- Souza, T.; Jennen, D.; van Delft, J.; van Herwijnen, M.; Kyrtoupolos, S.; Kleinjans, J. New insights into BaP-induced toxicity: Role of major metabolites in transcriptomics and contribution to hepatocarcinogenesis. Arch Toxicol 2016, 90, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Gelboin, H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [Green Version]
- Okano, P.; Miller, H.N.; Robinson, R.C.; Gelboin, H.V. Comparison of benzo(a)pyrene and (-)-trans-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene metabolism in human blood monocytes and lymphocytes. Cancer Res. 1979, 39, 3184–3193. [Google Scholar] [PubMed]
- Nebert, D.W.; Shi, Z.; Gálvez-Peralta, M.; Uno, S.; Dragin, N. Oral benzo[a]pyrene: Understanding pharmacokinetics, detoxication, and consequences--Cyp1 knockout mouse lines as a paradigm. Mol. Pharmacol. 2013, 84, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelkonen, O.; Nebert, D.W. Metabolism of polycyclic aromatic hydrocarbons: Etiologic role in carcinogenesis. Pharm. Rev. 1982, 34, 189–222. [Google Scholar]
- Kim, J.H.; Stansbury, K.H.; Walker, N.J.; Trush, M.A.; Strickland, P.T.; Sutter, T.R. Metabolism Of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis 1998, 19, 1847–1853. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, B. Relationships between Stress Granules, Oxidative Stress, and Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2017, 2017, 1809592. [Google Scholar] [CrossRef] [Green Version]
- Fang, A.H.; Smith, W.A.; Vouros, P.; Gupta, R.C. Identification and characterization of a novel benzo[a]pyrene-derived DNA adduct. Biochem. Biophys Res. Commun. 2001, 281, 383–389. [Google Scholar] [CrossRef]
- Baez, M.V.; Cercato, M.C.; Jerusalinsky, D.A. NMDA Receptor Subunits Change after Synaptic Plasticity Induction and Learning and Memory Acquisition. Neural Plast 2018, 2018, 5093048. [Google Scholar] [CrossRef]
- Castellano, C.; Cestari, V.; Ciamei, A. NMDA receptors and learning and memory processes. Curr. Drug Targets 2001, 2, 273–283. [Google Scholar] [CrossRef]
- Stephenson, F.A. Subunit characterization of NMDA receptors. Curr. Drug Targets 2001, 2, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.-L.; Tu, B.-J.; Yang, K.; Mo, T.-T.; Zhang, R.-Y.; Cheng, S.-Q.; Chen, C.-Z.; Jiang, X.-J.; Han, T.-L.; et al. Integrated Epigenetics, Transcriptomics, and Metabolomics to Analyze the Mechanisms of Benzo[a]pyrene Neurotoxicity in the Hippocampus. Toxicol. Sci. 2018, 166, 65–81. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherif, L.S.; Cao-Lei, L.; Farinelle, S.; Muller, C.P.; Turner, J.D.; Schroeder, H.; Grova, N. Assessment of 9-OH- and 7,8-diol-benzo[a]pyrene in Blood as Potent Markers of Cognitive Impairment Related to benzo[a]pyrene Exposure: An Animal Model Study. Toxics 2021, 9, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9030050
Cherif LS, Cao-Lei L, Farinelle S, Muller CP, Turner JD, Schroeder H, Grova N. Assessment of 9-OH- and 7,8-diol-benzo[a]pyrene in Blood as Potent Markers of Cognitive Impairment Related to benzo[a]pyrene Exposure: An Animal Model Study. Toxics. 2021; 9(3):50. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9030050
Chicago/Turabian StyleCherif, Lynda Saber, Lei Cao-Lei, Sophie Farinelle, Claude P. Muller, Jonathan D. Turner, Henri Schroeder, and Nathalie Grova. 2021. "Assessment of 9-OH- and 7,8-diol-benzo[a]pyrene in Blood as Potent Markers of Cognitive Impairment Related to benzo[a]pyrene Exposure: An Animal Model Study" Toxics 9, no. 3: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9030050