Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons
Abstract
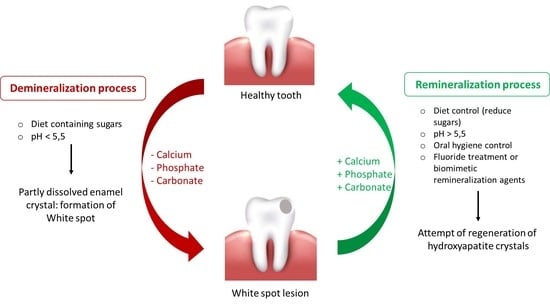
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
2.3. Inclusion Criteria
2.4. Data Processing
3. Results
4. Discussion
4.1. Casein-Phosphopeptide-Based Studies
4.2. Tricalcium Phosphate Studies
4.3. Self-Assembling Peptide 11-4 Studies
4.4. Nano-Hydroxyapatite Studies
4.5. Ozone Therapy Studies
4.6. Fluoride Agent Studies
4.7. Sealant Agent Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPP-ACFP | Casein phosphopeptide amorphous calcium fluoride phosphate |
| HA | Hydroxyapatite |
| ICON | Caries infiltrant |
| MIPP | MI Paste Plus |
| MIV | MI Varnish |
| SAP11-4 | Self-assembling peptide p11-4 |
| TCPF | Fluoride varnish with therapeutic tricalcium phosphate formulas |
| TCS | Tricalcium silicate paste |
| WSL | White spot lesion |
| WSLs | White spot lesions |
References
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-Doped Amorphous Calcium Phosphate Nanoparticles as a Promising Biomimetic Material for Dental Remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–Remineralization Dynamics in Teeth and Bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Inchingolo, F.; Charitos, I.A.; Cosola, M.D.; Cazzolla, A.P. Focus on the Cariogenic Process: Microbial and Biochemical Interactions with Teeth and Oral Environment. J. Biol. Regul. Homeost. Agents 2021, 35, 429–440. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Xu, C.; Yao, X.; Walker, M.P.; Wang, Y. Chemical/Molecular Structure of the Dentin-Enamel Junction Is Dependent on the Intratooth Location. Calcif. Tissue Int. 2009, 84, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradian-Oldak, J. The Regeneration of Tooth Enamel. Dimens. Dent. Hyg. 2009, 7, 12–15. [Google Scholar]
- Farooq, I.; Bugshan, A. The Role of Salivary Contents and Modern Technologies in the Remineralization of Dental Enamel: A Narrative Review. F1000Research 2021, 9, 171. [Google Scholar] [CrossRef]
- Angelova Volponi, A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Holmen, L.; Thylstrup, A.; Øgaard, B.; Kragh, F. A Scanning Electron Microscopic Study of Progressive Stages of Enamel Caries in Vivo. CRE 1985, 19, 355–367. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Borges, A.B.; Torres, L.M.S.; Gomes, I.S.; de Oliveira, R.S. Effect of Caries Infiltration Technique and Fluoride Therapy on the Colour Masking of White Spot Lesions. J. Dent. 2011, 39, 202–207. [Google Scholar] [CrossRef]
- Brodbelt, R.H.W.; O’brien, W.J.; Fan, P.L.; Frazer-Dib, J.G.; Yu, R. Translucency of Human Dental Enamel. J. Dent. Res. 1981, 60, 1749–1753. Available online: https://0-journals-sagepub-com.brum.beds.ac.uk/doi/10.1177/00220345810600100401?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 4 March 2023). [CrossRef] [Green Version]
- Houwink, B. The Index of Refraction of Dental Enamel Apatite. Br. Dent. J. 1974, 137, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological Perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Mahanti, M.; Koga, K.; Shitomi, K.; Miyaji, H. Rapid and Area-Specific Coating of Fluoride-Incorporated Apatite Layers by a Laser-Assisted Biomimetic Process for Tooth Surface Functionalization. Acta Biomater. 2018, 79, 148–157. Available online: https://0-reader-elsevier-com.brum.beds.ac.uk/reader/sd/pii/S1742706118304926?token=01A0A6391E49025102AF86C0010F1D73E4D9FC7F6ED91E1F1DBBCEDA434BB3DBDEEC320B357EE2F95CFAD32D06C11491&originRegion=eu-west-1&originCreation=20230218211059 (accessed on 18 February 2023). [CrossRef]
- Flemmig, T.F.; Beikler, T. Control of Oral Biofilms. Periodontology 2000 2011, 55, 9–15. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted Nano-Hydroxyapatite Toothpastes Reduce Biofilm Formation on Enamel and Resin-Based Composite Surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Converti, I.; Palermo, A.; Mancini, A.; Maggiore, M.; Tartaglia, G.; Ferrara, E.; Vecchiet, F.; Lorusso, F.; Scarano, A.; Bordea, R.; et al. Electric Toothbrush vs. Sonic Toothbrush, the Effectiveness on Gingival Inflammation: A Randomized Clinical Trial. J. Biol. Regul. Homeost. Agents 2022, 36, 205–215. [Google Scholar]
- Struzycka, I. The Oral Microbiome in Dental Caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, I.; Al-Thobity, A.M.; Al-Khalifa, K.S.; Alhooshani, K.; Sauro, S. An In-Vitro Evaluation of Fluoride Content and Enamel Remineralization Potential of Two Toothpastes Containing Different Bioactive Glasses. Biomed. Mater. Eng. 2020, 30, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Epple, M. Synthetic Hydroxyapatite as a Biomimetic Oral Care Agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Roveri, N.; Battistella, E.; Bianchi, C.L.; Foltran, I.; Foresti, E.; Iafisco, M.; Lelli, M.; Naldoni, A.; Palazzo, B.; Rimondini, L. Surface Enamel Remineralization: Biomimetic Apatite Nanocrystals and Fluoride Ions Different Effects. J. Nanomater. 2009, 2009, e746383. [Google Scholar] [CrossRef] [Green Version]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiriştioğlu, Z.; Yanikoglu, F.; Alkan, E.; Tagtekin, D. The Effect of Dental Paste with Herbal Content on Remineralization and the Imaging with Fluorescent Technique in Teeth with White Spot Lesion. Clin. Exp. Health Sci. 2021, 11, 348–353. [Google Scholar] [CrossRef]
- Marinelli, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Limongelli, L.; Montenegro, V.; Coloccia, G.; Laudadio, C.; Patano, A.; Inchingolo, F.; et al. White Spot Lesions in Orthodontics: Prevention and Treatment. A Descriptive Review. J. Biol. Regul. Homeost. Agents 2021, 35, 227–240. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Bharkhavy, K.V.; Shakir, A.; Augustine, D.; Sowmya, S.V.; Bahammam, H.A.; Bahammam, S.A.; Mohammad Albar, N.H.; Zidane, B.; Patil, S. The Anticariogenic Efficacy of Nano Silver Fluoride. Front. Bioeng. Biotechnol. 2022, 10, 931327. [Google Scholar] [CrossRef]
- Pasciuti, E.; Coloccia, G.; Inchingolo, A.D.; Patano, A.; Ceci, S.; Bordea, I.R.; Cardarelli, F.; Di Venere, D.; Inchingolo, F.; Dipalma, G. Deep Bite Treatment with Aligners: A New Protocol. Appl. Sci. 2022, 12, 6709. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, M.; Ma, Y.; Cao, L.; Xu, H.H.K.; Zhang, K.; Bai, Y. Effects of Fluoride and Calcium Phosphate Materials on Remineralization of Mild and Severe White Spot Lesions. BioMed Res. Int. 2019, 2019, e1271523. [Google Scholar] [CrossRef]
- Cury, J.A.; Tenuta, L.M.A. Enamel Remineralization: Controlling the Caries Disease or Treating Early Caries Lesions? Braz. Oral Res. 2009, 23 (Suppl. S1), 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Cabezas, C. The Chemistry of Caries: Remineralization and Demineralization Events with Direct Clinical Relevance. Dent. Clin. N. Am. 2010, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Warshawsky, H.; Nanci, A. Stereo Electron Microscopy of Enamel Crystallites. J. Dent. Res. 1982, 1504–1514. [Google Scholar]
- Cui, F.-Z.; Ge, J. New Observations of the Hierarchical Structure of Human Enamel, from Nanoscale to Microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U. The Hidden Structure of Human Enamel. Nat. Commun. 2019, 10, 4383. Available online: https://0-www-nature-com.brum.beds.ac.uk/articles/s41467-019-12185-7 (accessed on 18 February 2023). [CrossRef] [Green Version]
- Robinson, C.; Shore, R.C.; Brookes, S.J.; Strafford, S.; Wood, S.R.; Kirkham, J. The Chemistry of Enamel Caries. Crit. Rev. Oral Biol. Med. 2000, 11, 481–495. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Saito, M.; Tohei, T.; Takano, Y.; Ikuhara, Y. Fluorine in Shark Teeth: Its Direct Atomic-Resolution Imaging and Strengthening Function. Angew. Chem. Int. Ed. 2014, 53, 1543–1547. [Google Scholar] [CrossRef]
- Vaughn, J.S.; Woerner, W.R.; Lindsley, D.H.; Nekvasil, H.; Hughes, J.M.; Phillips, B.L. Hydrogen Environments In Low-OH, F, Cl Apatites Revealed By Double Resonance Solid-State NMR. J. Phys. Chem. C 2015, 119, 28605–28613. [Google Scholar] [CrossRef]
- Manjubala, I.; Sivakumar, M.; Nikkath, S.N. Synthesis and Characterisation of Hydroxy/Fluoroapatite Solid Solution. J. Mater. Sci. 2001, 36, 5481–5486. [Google Scholar] [CrossRef]
- Aoba, T. The Effect of Fluoride On Apatite Structure and Growth. Crit. Rev. Oral Biol. Med. 1997, 8, 136–153. [Google Scholar] [CrossRef] [Green Version]
- Marquis, R.E. Antimicrobial Actions of Fluoride for Oral Bacteria. Can. J. Microbiol. 1995, 41, 955–964. Available online: https://cdnsciencepub.com/doi/10.1139/m95-133 (accessed on 18 February 2023). [CrossRef] [PubMed]
- Al-Batayneh, O.B.; Bani Hmood, E.I.; Al-Khateeb, S.N. Assessment of the Effects of a Fluoride Dentifrice and GC Tooth Mousse on Early Caries Lesions in Primary Anterior Teeth Using Quantitative Light-Induced Fluorescence: A Randomised Clinical Trial. Eur. Arch. Paediatr. Dent. 2020, 21, 85–93. [Google Scholar] [CrossRef]
- Badiee, M.; Jafari, N.; Fatemi, S.; Ameli, N.; Kasraei, S.; Ebadifar, A. Comparison of the Effects of Toothpastes Containing Nanohydroxyapatite and Fluoride on White Spot Lesions in Orthodontic Patients: A Randomized Clinical Trial. Dent. Res. J. 2020, 17, 354–359. [Google Scholar]
- Kau, C.H.; Wang, J.; Palombini, A.; Abou-Kheir, N.; Christou, T. Effect of Fluoride Dentifrices on White Spot Lesions during Orthodontic Treatment: A Randomized Trial. Angle Orthod. 2019, 89, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of Nano-Hydroxyapatite and Ozone on Approximal Initial Caries: A Randomized Clinical Trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef]
- Handa, A.; Chengappa, D.; Sharma, P.; Handa, J.K. Effectiveness of Clinpro Tooth Crème in Comparison with MI Varnish with RECALDENTTM for Treatment of White Spot Lesions: A Randomized Controlled Trial. Clin. Oral Investig. 2022. [Google Scholar] [CrossRef] [PubMed]
- Heravi, F.; Ahrari, F.; Tanbakuchi, B. Effectiveness of MI Paste Plus and Remin Pro on Remineralization and Color Improvement of Postorthodontic White Spot Lesions. Dent. Res. J. 2018, 15, 95–103. [Google Scholar]
- Kobeissi, R.; Badr, S.B.; Osman, E. Effectiveness of Self-Assembling Peptide P11-4 Compared to Tricalcium Phosphate Fluoride Varnish in Remineralization of White Spot Lesions: A Clinical Randomized Trial. Int. J. Clin. Pediatr. Dent. 2020, 13, 451–456. [Google Scholar] [CrossRef]
- AlFeel, J.; Laflouf, M.; AlKurdi, S.; Alkhouli, M. Evaluating the Effect of Clinpro Tooth Crème on Remineralization of Pre-Carious White Spot Lesions in Anterior Primary Teeth: Randomized Controlled Clinical Trial. Pediatr. Dent. J. 2021, 31, 152–158. [Google Scholar] [CrossRef]
- Aboulnaga, M.A.; Akah, M.M.; Hassanein, O.E.-S. Evaluation of Remineralization Potential of Remin Pro Forte vs Remin Pro on White Spot Lesions: A Randomized Clinical Trial. J. Contemp. Dent. Pract. 2022, 23, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, K.; Hamama, H.H.; Motawea, A.; Fawzy, A.; Mahmoud, S.H. Long-Term Evaluation of Early-Enamel Lesions Treated with Novel Experimental Tricalcium Silicate Paste: A 2-Year Randomized Clinical Trial. J. Esthet. Restor. Dent. 2022, 34, 1113–1121. [Google Scholar] [CrossRef]
- Beerens, M.W.; ten Cate, J.M.; Buijs, M.J.; van der Veen, M.H. Long-Term Remineralizing Effect of MI Paste Plus on Regression of Early Caries after Orthodontic Fixed Appliance Treatment: A 12-Month Follow-up Randomized Controlled Trial. Eur. J. Orthod. 2018, 40, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Rechmann, P.; Bekmezian, S.; Rechmann, B.M.T.; Chaffee, B.W.; Featherstone, J.D.B. MI Varnish and MI Paste Plus in a Caries Prevention and Remineralization Study: A Randomized Controlled Trial. Clin. Oral Investig. 2018, 22, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Massaro, C.; Buzalaf, M.A.R.; Janson, G.; Garib, D. Prevention of Non-Cavitated Lesions with Fluoride and Xylitol Varnishes during Orthodontic Treatment: A Randomized Clinical Trial. Clin. Oral Investig. 2021, 25, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Bröseler, F.; Tietmann, C.; Bommer, C.; Drechsel, T.; Heinzel-Gutenbrunner, M.; Jepsen, S. Randomised Clinical Trial Investigating Self-Assembling Peptide P11-4 in the Treatment of Early Caries. Clin. Oral Investig. 2020, 24, 123–132. [Google Scholar] [CrossRef]
- Alsabek, L.; Al-Nerabieah, Z.; Bshara, N.; Comisi, J.C. Retention and Remineralization Effect of Moisture Tolerant Resin-Based Sealant and Glass Ionomer Sealant on Non-Cavitated Pit and Fissure Caries_ Randomized Controlled Clinical Trial. J. Dent. 2019, 86, 69–74. Available online: https://0-reader-elsevier-com.brum.beds.ac.uk/reader/sd/pii/S0300571219301101?token=1A3AFCFF15E7DFEF990894D5741DD5027439B025EAA97950349729742CE154CADF6011C3381ECAC1E8929D3979B9FA28&originRegion=eu-west-1&originCreation=20230215211312 (accessed on 15 February 2023). [CrossRef]
- Flynn, L.N.; Julien, K.; Noureldin, A.; Buschang, P.H. The Efficacy of Fluoride Varnish vs a Filled Resin Sealant for Preventing White Spot Lesions during Orthodontic Treatment: A randomized clinical trial. Angle Orthod. 2022, 92, 204–212. [Google Scholar] [CrossRef]
- Baafif, H.A.; Alibrahim, I.F.; Alotaibi, S.H.; Alharbi, H.G.; Shubaily, M.N.; Elkwatehy, W.M.A. The Efficacy of Resin Infiltrant and Casein Phosphopeptide–Amorphous Calcium Fluoride Phosphate in Treatment of White Spot Lesions (Comparative Study). J. Int. Soc. Prev. Community Dent. 2020, 10, 438–444. [Google Scholar] [CrossRef]
- Shen, P.; Bagheri, R.; Walker, G.D.; Yuan, Y.; Stanton, D.P.; Reynolds, C.; Reynolds, E.C. Effect of Calcium Phosphate Addition to Fluoride Containing Dental Varnishes on Enamel Demineralization. Aust. Dent. J. 2016, 61, 357–365. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/full/10.1111/adj.12385 (accessed on 18 February 2023). [CrossRef] [Green Version]
- Cross, K.J.; Huq, N.L.; Stanton, D.P.; Sum, M.; Reynolds, E.C. NMR Studies of a Novel Calcium, Phosphate and Fluoride Delivery Vehicle-Alpha(S1)-Casein(59-79) by Stabilized Amorphous Calcium Fluoride Phosphate Nanocomplexes. Biomaterials 2004, 25, 5061–5069. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Saranathan, S.; Cai, F.; Cross, K.J.; Reynolds, E.C. Enamel Subsurface Lesion Remineralisation with Casein Phosphopeptide Stabilised Solutions of Calcium, Phosphate and Fluoride. Caries Res. 2008, 42, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Kavitha, M.; Loganathan, S.C. Comparison of the Remineralization Potential of CPP-ACP and CPP-ACP with 900 Ppm Fluoride on Eroded Human Enamel: An in Situ Study. Arch. Oral Biol. 2010, 55, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-Assembling Peptide Scaffolds Promote Enamel Remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Combined Effects of Nano-Hydroxyapatite and Galla Chinensis on Remineralisation of Initial Enamel Lesion in Vitro. J. Dent. 2010, 38, 811–819. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D.; Kim, B.I. Remineralization Potential of New Toothpaste Containing Nano-Hydroxyapatite. Key Eng. Mater 2006, 309–311 I, 537–540. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-Hydroxyapatite and Its Applications in Preventive, Restorative and Regenerative Dentistry: A Review of Literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Baysan, A.; Whiley, R.A.; Lynch, E. Antimicrobial Effect of a Novel Ozone-Generating Device on Micro-Organisms Associated with Primary Root Carious Lesions in Vitro. Caries Res. 2000, 34, 498–501. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Limeback, H.; Lawrence, H.P.; Fillery, E.D. Evaluating the Effect of an Ozone Delivery System on the Reversal of Dentin Hypersensitivity: A Randomized, Double-Blinded Clinical Trial. J. Endod. 2009, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Dähnhardt, J.E.; Jaeggi, T.; Lussi, A. Treating Open Carious Lesions in Anxious Children with Ozone. A Prospective Controlled Clinical Study. Am. J. Dent. 2006, 19, 267–270. [Google Scholar] [PubMed]
- Ballini, A.; Cantore, S.; Signorini, L.; Saini, R.; Scacco, S.; Gnoni, A.; Inchingolo, A.D.; De Vito, D.; Santacroce, L.; Inchingolo, F.; et al. Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 18, 44. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Saini, R.; Pettini, F.; Fotopoulou, E.A.; Saini, S.R.; Georgakopoulos, I.P.; Dipalma, G.; Gargiulo Isacco, C.; Inchingolo, F. Effect of Activated Charcoal Probiotic Toothpaste Containing Lactobacillus Paracasei and Xylitol on Dental Caries: A Randomized and Controlled Clinical Trial. J. Biol. Regul. Homeost. Agents 2019, 33, 977–981. [Google Scholar] [PubMed]
- Kharouf, N.; Sauro, S.; Eid, A.; Zghal, J.; Jmal, H.; Seck, A.; Macaluso, V.; Addiego, F.; Inchingolo, F.; Affolter-Zbaraszczuk, C.; et al. Physicochemical and Mechanical Properties of Premixed Calcium Silicate and Resin Sealers. J. Funct. Biomater. 2022, 14, 9. [Google Scholar] [CrossRef] [PubMed]




| Authors | Type of Study | Object | Study Design and Timeline | Results |
|---|---|---|---|---|
| O. B. Al-Batayneh, 2019 [43] | Randomized Clinical Trial | Effects of fluoride dentifrice and GC Tooth Mousse on early caries lesions. | 114 children used three different agents twice daily: fluoride dentifrice (500 ppm), CPP-ACP Crème, and fluoride dentifrice + CPP-ACP Crème. Lesions compared at baseline, 3 and 6 month after. | The use of both agents provided no additional advantage over their solo usage. |
| Mohammadreza Badiee, 2019 [44] | Randomized Clinical Trial | Comparison of the effects of toothpastes containing nanohydroxyapatite and fluoride on white spots in orthodontic patients. | After orthodontic treatment, 50 patients used toothpaste containing fluoride or nano-hydroxyapatite. Follow-up: 1, 3 and 6 months after. | Nanohydroxyapatite toothpaste performed better than the other containing fluoride. |
| Chung H. Kau, 2019 [45] | Randomized Trial | Effects of three different fluoride dentifrices on white spot lesions during orthodontic treatment. | 120 patients used three types of fluoride dentifrices (Clinpro 5000, Clinpro Tooth Crème and MI-Paste Plus) twice a day for 4 months. | Clinpro 5000 performs somewhat better than the other two test pastes. |
| Katarzyna Grocholewicz, 2020 [46] | Randomized Clinical Trial | Effect of nano-hydroxyapatite and ozone on approximal initial caries. | Comparison of three methods (a nano-hydroxyapatite gel, gaseous ozone therapy, combination of the previous two) for enamel remineralization in 92 patients with initial approximal lesions. Follow-up: 1, 2 years after. | The combination of both methods produced the best effects. |
| Ashish Handa, 2022 [47] | Randomized Controlled Trial | Comparison between Clinpro Tooth Creème and MI Varnish with RECALDENTTM for treatment of white spot lesions. | 35 patients divided into three groups: ClinproTM Tooth Creème group, Fluoride varnish group, home-care group (control). | Clinpro Tooth Crème outperforms MI Varnish with RECALDENTTM (CPP-ACP) in terms of enamel decalcification protection. |
| Farzin Heravi, 2018 [48] | Randomized Clinical Trial | Comparison between MI Paste Plus and Remin Pro on the remineralization of white spot lesions in postorthodontic patients. | 39 patients with white spot lesions divided into three treatment groups: MI Paste Plus group, Premin Pro group, control group. Follow-up: 4, 8 and 12 weeks later. | The use of both agents helped reduce post-orthodontic white spot lesions. |
| Riham Kobeissi, 2020 [49] | Randomized Clinical Trial | Compare the efficacy of SAP11-4 vs. tricalcium phosphate fluoride (TCPF) in remineralizing WSLs in young permanent teeth. | Nine patients received either TCPF (group 1) or SAP11-4 (group 2). Follow-up: baseline, 3 and 6 months later. | Greater effectiveness of SAP11-4 compared with TCPF in treating white spots. |
| AlFeel, 2021 [50] | Randomized Controlled Clinical Trial | Effect of Clinpro Tooth Crème on remineralization of white spot lesions. | Split-mouth study: 18 patients applied Clinpro Tooth Crème on one side and no treatment on the other side. | Clinpro tooth crème has a remineralizing action on white spot lesions compared to normal oral hygiene. |
| Aboulnaga, 2022 [51] | Randomized Clinical Trial | Effect of Remin Pro and Remin Pro Forte on white spot lesions in orthodontic patients. | 20 patients divided into two groups (Remin pro group and Remin pro forte group) were followed for 3 months. | The use of Remin Pro Forte provided greater benefits than the use of Remin Pro. |
| Hamdi, 2022 [52] | Randomized Clinical trial | Remineralizing potential of experimental tricalcium silicate paste (TCS) in comparison with CPP-ACP and SDF-KI. | 45 patients divided into three groups (TCS, SDF-KI and CPP-ACP group). Follow-up periods: 3,6,12 and 24 months. | TCS showed potential remineralization of white spot lesions. |
| Beerens, 2018 [53] | Randomized Controlled trial | Remineralizing effect of MI Paste Plus (MPP) on white spot lesions after orthodontic treatments. | Long-term effect (12 months) of MPP versus a placebo paste in 65 partecipants. | The use of MPP in post-orthodontic patients did not improve the white spot lesions. |
| Rechmann, 2018 [54] | Randomized Controlled Trial | Effects of MI Paste Plus (MIPP) and MI Varnish (MIV) on white spot lesions in orthodontic patients. | 40 patients randomly assigned to the experimental group (MIPP or MIV) or the control group. | No significant difference between MIPP and MIV. |
| Silva, 2021 [55] | Randomized Clinical Trial | Effects of fluoride and xylitol varnishes during orthodontic treatment. | Fluoride, xylitol varnish or placebo were applied in 55 orthodontic patients. Follow-up: baseline and 6 months after. | In short term, both varnishes produce remineralization. |
| Broseler, 2020 [56] | Randomized Clinical Trial | Efficacy of self-assembling peptide P11-4 and fluoride varnish in white spot lesions. | 37 subject treated with P11-4 (test group) or fluoride varnish (control group). Follow-up: 1 year. | Early carious lesions treated with P11-4 were reduced. |
| Alsabek, 2019 [57] | Randomized Controlled Clinical Trial | Remineralization effect of resin-based sealant and glass ionomer sealent on non-cavitated pit and fissure caries. | Split mouse study on 40 patients: moisture tolerant sealant was applied on one side of the mouth and glass ionomer sealant was applied on the other side. | Both agents have demonstrated remineralizing capacity of pit and fissure caries. |
| Flynn, 2022 [58] | Randomized Clinical Trial | Efficacy of fluoride varnish vs. a filled resin sealant for preventing white spot lesions in orthodontic patients. | 40 orthodontic patients divided into two groups: sealent group (application every 3 months); MI Varnish group (application every 4-6 weeks). Follow up: 12 months. | Similar levels of protection by both agents. |
| Baafif, 2020 [59] | Comparative Study | Efficacy of ICON vs. CPP-ACFP. | Split-mouth technique: 30 patients have been treated with ICON on the left side and with CPP-ACFP on the right side. | The efficacy of CPP-ACFP was better than ICON. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering10040472
Malcangi G, Patano A, Morolla R, De Santis M, Piras F, Settanni V, Mancini A, Di Venere D, Inchingolo F, Inchingolo AD, et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering. 2023; 10(4):472. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering10040472
Chicago/Turabian StyleMalcangi, Giuseppina, Assunta Patano, Roberta Morolla, Matteo De Santis, Fabio Piras, Vito Settanni, Antonio Mancini, Daniela Di Venere, Francesco Inchingolo, Alessio Danilo Inchingolo, and et al. 2023. "Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons" Bioengineering 10, no. 4: 472. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering10040472