Characteristics and Resistance to Cisplatin of Human Neuroblastoma Cells Co-Cultivated with Immune and Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
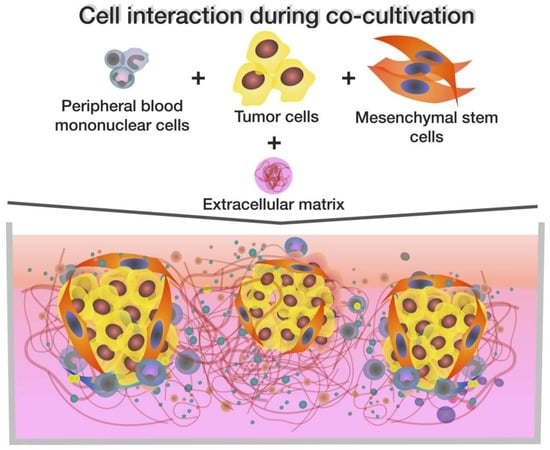
2.1. Cell Cultures
2.2. Immunophenotyping and Differentiation of BM-MSCs
2.3. Obtaining of GFP-Modified SH-SY5Y Cells
2.4. Cell Labeling
2.5. Preparation of Matrigel-Coated Plates
2.6. Fluorescence and Confocal Microscopy
2.7. Fluorescence-Activated Cell Sorting (FACS) of Cells from Double and Triple Co-Cultures
2.8. Cytokine Profile
2.9. Cell Proliferation Assay
2.10. Quantitative Polymerase Chain Reaction (qPCR)
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. BM-MSCs, SH-SY5Y Cells, and PBMCs Interact in Double and Triple Co-Cultures, Vesicular Transport, and Exchange of the Membrane and Cytoplasmic Components
3.2. Co-Cultivation Leads to Changes in the Cytokine Profile of BM-MSCs, SH-SY5Y Cells, and PBMCs
3.3. Co-Culture of BM-MSCs, SH-SY5Y Cells, and PBMCs Is more Resistant to the Cytotoxic Effect of Cisplatin Than the Co-Culture of BM-MSCs and SH-SY5Y Cells
3.4. Cisplatin Treatment and Co-Cultivation Change Levels of BAX, BCL2, BCL2L1, CAV1, CASP3, and RAC1 Gene Expression in SH-SY5Y
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jeong, C.H.; Woo, J.S.; Ryu, C.H.; Lee, J.H.; Jeun, S.S. In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: Tropism for brain tumors and biodistribution. Int. J. Nanomed. 2016, 11, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Y.; Wang, Z.; Gutkind, J.S.; Wang, Z.; Wang, F.; Lu, J.; Niu, G.; Teng, G.; Chen, X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells 2015, 33, 456–467. [Google Scholar] [CrossRef]
- Trivanovic, D.; Krstic, J.; Djordjevic, I.O.; Mojsilovic, S.; Santibanez, J.F.; Bugarski, D.; Jaukovic, A. The roles of mesenchymal stromal/stem cells in tumor microenvironment associated with inflammation. Mediat. Inflamm. 2016, 2016, 7314016. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (tam)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [Green Version]
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 2019, 133, 2159–2167. [Google Scholar] [CrossRef]
- Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Cell culture based in vitro test systems for anticancer drug screening. Front. Bioeng. Biotechnol. 2020, 8, 322. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Solovyeva, V.V.; James, V.; Arkhipova, S.S.; Gomzikova, M.O.; Garanina, E.E.; Akhmetzyanova, E.R.; Tazetdinova, L.G.; Khaiboullina, S.F.; Rizvanov, A.A. Human mesenchymal stem cells overexpressing interleukin 2 can suppress proliferation of neuroblastoma cells in co-culture and activate mononuclear cells in vitro. Bioengineering 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Kitaeva, K.V.; Prudnikov, T.S.; Gomzikova, M.O.; Kletukhina, S.K.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Analysis of the interaction and proliferative activity of adenocarcinoma, peripheral blood mononuclear and mesenchymal stromal cells after co-cultivation in vitro. BioNanoScience 2019, 9, 502–509. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Kitaeva, K.V.; Chulpanova, D.S.; Arkhipova, S.S.; Filin, I.Y.; Rizvanov, A.A. Analysis of the interaction of human neuroblastoma cell-derived cytochalasin b induced membrane vesicles with mesenchymal stem cells using imaging flow cytometry. BioNanoScience 2022, 12, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Rizvanov, A.A.; Yalvac, M.E.; Shafigullina, A.K.; Salafutdinov, I.; Blatt, N.L.; Sahin, F.; Kiyasov, A.P.; Palotas, A. Interaction and self-organization of human mesenchymal stem cells and neuro-blastoma sh-sy5y cells under co-culture conditions: A novel system for modeling cancer cell micro-environment. Eur. J. Pharm. Biopharm. 2010, 76, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The nci60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Elliott, N.T.; Yuan, F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 2010, 100, 59–74. [Google Scholar] [CrossRef]
- Galateanu, B.; Hudita, A.; Negrei, C.; Ion, R.M.; Costache, M.; Stan, M.; Nikitovic, D.; Hayes, A.W.; Spandidos, D.A.; Tsatsakis, A.M.; et al. Impact of multicellular tumor spheroids as an in vivolike tumor model on anticancer drug response. Int. J. Oncol. 2016, 48, 2295–2302. [Google Scholar] [CrossRef] [Green Version]
- Probert, C.; Dottorini, T.; Speakman, A.; Hunt, S.; Nafee, T.; Fazeli, A.; Wood, S.; Brown, J.E.; James, V. Communication of prostate cancer cells with bone cells via extracellular vesicle rna; a potential mechanism of metastasis. Oncogene 2018, 38, 1751–1763. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Gilazieva, Z.E.; Kletukhina, S.K.; Aimaletdinov, A.M.; Garanina, E.E.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Cytochalasin b-induced membrane vesicles from human mesenchymal stem cells overexpressing il2 are able to stimulate cd8+ t-killers to kill human triple negative breast cancer cells. Biology 2021, 10, 141. [Google Scholar] [CrossRef]
- Chowdhury, R.; Webber, J.P.; Gurney, M.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2015, 6, 715–731. [Google Scholar] [CrossRef]
- Mandel, K.; Yang, Y.; Schambach, A.; Glage, S.; Otte, A.; Hass, R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev. 2013, 22, 3114–3127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bucan, V.; Baehre, H.; von der Ohe, J.; Otte, A.; Hass, R. Acquisition of new tumor cell properties by msc-derived exosomes. Int. J. Oncol. 2015, 47, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Hong, I.S.; Lee, H.Y.; Kang, K.S. Mesenchymal stem cells and cancer: Friends or enemies? Mutat. Res. 2014, 768, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.P.; Luetzkendorf, J.; Mueller, T.; Reichelt, K.; Simon, H.; Schmoll, H.J. Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: Evidence of resistance to apoptosis induction. Stem Cells 2006, 24, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, B.C.; Abreu, B.R.; Grivicich, I.; Markarian, C.F.; Chem, E.; Camassola, M.; Nardi, N.B.; Dihl, R.R. Human mesenchymal stem cells are resistant to cytotoxic and genotoxic effects of cisplatin in vitro. Genet. Mol. Biol. 2016, 39, 129–134. [Google Scholar] [CrossRef]
- Gilazieva, Z.E.; Tazetdinova, L.G.; Arkhipova, S.S.; Solovyeva, V.V.; Rizvanov, A.A. Effect of cisplatin on ultrastructure and viability of adipose-derived mesenchymal stem cells. BioNanoScience 2016, 6, 534–539. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Skolekova, S.; Matuskova, M.; Bohac, M.; Toro, L.; Durinikova, E.; Tyciakova, S.; Demkova, L.; Gursky, J.; Kucerova, L. Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells. Cell Commun. Signal. CCS 2016, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Wang, Z.; Zhong, Y.; Lan, J.; Li, X.; Lin, H. Ccr9-ccl25 interaction suppresses apoptosis of lung cancer cells by activating the pi3k/akt pathway. Med. Oncol. 2015, 32, 66. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Xia, W.; Hou, M. Macrophage migration inhibitory factor rescues mesenchymal stem cells from doxorubicin-induced senescence though the pi3k-akt signaling pathway. Int. J. Mol. Med. 2018, 41, 1127–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.F.; Huang, Y.; Xu, C.L.; Lin, L.Y.; Han, Y.Y.; Sun, W.H.; Hu, G.H.; Rabson, A.B.; Wang, Y.; Shi, Y.F. Downregulation of cxcl12 in mesenchymal stromal cells by tgfbeta promotes breast cancer metastasis. Oncogene 2017, 36, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Fang, Y.; Henderson, F.C., Jr.; Yi, Q.; Lei, Q.; Li, Y.; Chen, N. Chemokine cxcl16 expression suppresses migration and invasiveness and induces apoptosis in breast cancer cells. Mediat. Inflamm. 2014, 2014, 478641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensbergen, P.J.; Wijnands, P.G.; Schreurs, M.W.; Scheper, R.J.; Willemze, R.; Tensen, C.P. The cxcr3 targeting chemokine cxcl11 has potent antitumor activity in vivo involving attraction of cd8+ t lymphocytes but not inhibition of angiogenesis. J. Immunother. 2005, 28, 343–351. [Google Scholar] [CrossRef]
- Namkoong, H.; Song, M.Y.; Seo, Y.B.; Choi, D.H.; Kim, S.W.; Im, S.J.; Sung, Y.C.; Park, Y. Enhancement of antigen-specific cd8 t cell responses by co-delivery of fc-fused cxcl11. Vaccine 2014, 32, 1205–1212. [Google Scholar] [CrossRef]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E.; et al. An alternatively spliced variant of cxcr3 mediates the inhibition of endothelial cell growth induced by ip-10, mig, and i-tac, and acts as functional receptor for platelet factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef]
- Zhai, J.; Shen, J.; Xie, G.; Wu, J.; He, M.; Gao, L.; Zhang, Y.; Yao, X.; Shen, L. Cancer-associated fibroblasts-derived il-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019, 454, 37–43. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, L.; Luo, G.; Son, H.; Prectoni, J.H.; Zheng, W. Effect of the cytokine levels in serum on osteosarcoma. Tumour Biol. 2014, 35, 1023–1028. [Google Scholar] [CrossRef]
- Higashino, N.; Koma, Y.I.; Hosono, M.; Takase, N.; Okamoto, M.; Kodaira, H.; Nishio, M.; Shigeoka, M.; Kakeji, Y.; Yokozaki, H. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of ccl2 and interleukin-6 in esophageal squamous cell carcinoma. Lab. Invest. 2019, 99, 777–792. [Google Scholar] [CrossRef]
- Ebbing, E.A.; van der Zalm, A.P.; Steins, A.; Creemers, A.; Hermsen, S.; Rentenaar, R.; Klein, M.; Waasdorp, C.; Hooijer, G.K.J.; Meijer, S.L.; et al. Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc. Natl. Acad. Sci. USA 2019, 116, 2237–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Naour, A.; Prat, M.; Thibault, B.; Mével, R.; Lemaitre, L.; Leray, H.; Joubert, M.V.; Coulson, K.; Golzio, M.; Lefevre, L.; et al. Tumor cells educate mesenchymal stromal cells to release chemoprotective and immunomodulatory factors. J. Mol. Cell. Biol. 2020, 12, 202–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anton, K.; Banerjee, D.; Glod, J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased il-6 and cxcl10 secretion. PLoS ONE 2012, 7, e35036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Z.; Zhang, J.; Shi, Y.; Li, W.; Shi, H.; Ji, R.; Mao, F.; Qian, H.; Xu, W.; Zhang, X. Cxcl5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis 2020, 9, 63. [Google Scholar] [CrossRef]
- Renner, N.A.; Ivey, N.S.; Redmann, R.K.; Lackner, A.A.; MacLean, A.G. Mcp-3/ccl7 production by astrocytes: Implications for siv neuroinvasion and aids encephalitis. J. Neurovirol. 2011, 17, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Taschner-Mandl, S.; Schwarz, M.; Blaha, J.; Kauer, M.; Kromp, F.; Frank, N.; Rifatbegovic, F.; Weiss, T.; Ladenstein, R.; Hohenegger, M.; et al. Metronomic topotecan impedes tumor growth of mycn-amplified neuroblastoma cells in vitro and in vivo by therapy induced senescence. Oncotarget 2016, 7, 3571–3586. [Google Scholar] [CrossRef] [Green Version]
- Rupertus, K.; Sinistra, J.; Scheuer, C.; Nickels, R.M.; Schilling, M.K.; Menger, M.D.; Kollmar, O. Interaction of the chemokines i-tac (cxcl11) and sdf-1 (cxcl12) in the regulation of tumor angiogenesis of colorectal cancer. Clin. Exp. Metastasis 2014, 31, 447–459. [Google Scholar] [CrossRef]
- Kuehnemuth, B.; Piseddu, I.; Wiedemann, G.M.; Lauseker, M.; Kuhn, C.; Hofmann, S.; Schmoeckel, E.; Endres, S.; Mayr, D.; Jeschke, U.; et al. Ccl1 is a major regulatory t cell attracting factor in human breast cancer. BMC Cancer 2018, 18, 1278. [Google Scholar] [CrossRef] [Green Version]
- Ohue, Y.; Nishikawa, H. Regulatory t (treg) cells in cancer: Can treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [Green Version]
- Tahir, I.M.; Iqbal, T.; Jamil, A.; Saqib, M. Association of bcl-2 with oxidative stress and total antioxidant status in pediatric acute lymphoblastic leukemia. J. Biol. Regul. Homeost. Agents 2017, 31, 1023–1027. [Google Scholar]
- Correia, C.; Lee, S.H.; Meng, X.W.; Vincelette, N.D.; Knorr, K.L.; Ding, H.; Nowakowski, G.S.; Dai, H.; Kaufmann, S.H. Emerging understanding of bcl-2 biology: Implications for neoplastic progression and treatment. Biochim. Biophys. Acta 2015, 1853, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xie, Q.; Qi, L.; Wang, C.; Xu, N.; Liu, W.; Yu, Y.; Li, S.; Xu, Y. Bcl-2 overexpression reduces cisplatin cytotoxicity by decreasing er-mitochondrial ca2+ signaling in skov3 cells. Oncol. Rep. 2018, 39, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Fanfone, D.; Idbaih, A.; Mammi, J.; Gabut, M.; Ichim, G. Profiling anti-apoptotic bcl-xl protein expression in glioblastoma tumorspheres. Cancers 2020, 12, 2853. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.L.; Huang, Q.; Liu, X.; Li, F.; Zimmerman, M.A.; Li, C.Y. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. J. Invest. Dermatol. 2014, 134, 1686–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, A.; Chevrier, S.; Beltjens, F.; Dosset, M.; Viltard, E.; Lagrange, A.; Derangere, V.; Oudot, A.; Ghiringhelli, F.; Collin, B.; et al. Cleaved caspase-3 transcriptionally regulates angiogenesis-promoting chemotherapy resistance. Cancer Res. 2019, 79, 5958–5970. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Kashyap, T.; Mishra, P.; Panda, A.K.; Nagini, S.; Mishra, R. Role and regulation of proapoptotic bax in oral squamous cell carcinoma and drug resistance. Head Neck 2019, 41, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, L. Effect of drug-loaded microbubbles combined with ultrasound on the apoptosis of cancer cells and the expression of bax and bcl-2 in a rabbit vx2 liver tumor model. Biosci. Rep. 2019, 39, BSR20181144. [Google Scholar] [CrossRef] [Green Version]
- Velinovic, M.; Jankovic, R.; Jovanovic, D.; Skodric Trifunovic, V.; Gavrilovic, D.; Stojsic, J.; Cavic, M. Tumor characteristics, expressions of ercc1, bax, p53, igf1r, bcl2, bcl2/bax and prognostic factors for overall survival in patients with lung carcinoid. J. BUON 2019, 24, 256–266. [Google Scholar]
- Akay, I.; Oxmann, D.; Helfenstein, A.; Mentlein, R.; Schunke, M.; Hassenpflug, J.; Kurz, B. Tumor risk by tissue engineering: Cartilaginous differentiation of mesenchymal stem cells reduces tumor growth. Osteoarthr. Cartil. 2010, 18, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Koleske, A.J.; Baltimore, D.; Lisanti, M.P. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc. Natl. Acad. Sci. USA 1995, 92, 1381–1385. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Valdivia, N.I.; Calderon, C.C.; Diaz, J.E.; Lobos-Gonzalez, L.; Sepulveda, H.; Ortiz, R.J.; Martinez, S.; Silva, V.; Maldonado, H.J.; Silva, P.; et al. Anti-neoplastic drugs increase caveolin-1-dependent migration, invasion and metastasis of cancer cells. Oncotarget 2017, 8, 111943–111965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez del Pulgar, T.; Bandres, E.; Espina, C.; Valdes-Mora, F.; Perez-Palacios, R.; Garcia-Amigot, F.; Garcia-Foncillas, J.; Lacal, J.C. Differential expression of rac1 identifies its target genes and its contribution to progression of colorectal cancer. Int. J. Biochem. Cell Biol. 2007, 39, 2289–2302. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Cho, S.J.; Chang, K.K.; Park, D.J.; Ryeom, S.W.; Yoon, S.S. Role of rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol. Cancer Res. MCR 2017, 15, 1106–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajcini, K.V.; Pomerantz, J.H.; Alkan, O.; Doyonnas, R.; Blau, H.M. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J. Cell Biol. 2008, 180, 1005–1019. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef]








| Target Gene | Forward Primer (5′−3′) | Reverse Primer (5′−3′) | TaqMan Probe (5′−3′) |
|---|---|---|---|
| 18S rRNA | GCCGCTAGAGGTGAAATTCTTG | CATTCTTGGCAAATGCTTTCG | [HEX] ACCGGCGCAAGACGGACCAG [BH2] |
| BCL2 | GTGGATGACTGAGTACCTGAAC | GCCAGGAGAAATCAAACAGAGG | [FAM] CAGGATAACGGAGGCTGGGATGC [BH1] |
| BCL2L1 | GACATCCCAGCTCCACATC | GTTCCCATAGAGTTCCACAAAAG | [FAM] CCCCAGGGACAGCATATCAGAGC [BH1] |
| RAC1 | GGTGAATCTGGGCTTATGGG | TCAGGATACCACTTTGCACG | [FAM] TTTGCTTTTCCCTTGTGAGTCCTGC [BH1] |
| CAV1 | CCTTCCTCAGTTCCCTTAAAGC | TGTAGATGTTGCCCTGTTCC | [FAM] TCCTCACAGTTTTCATCCAGCCACG [BH1] |
| CASP3 | CCTACAGCCCATTTCTCCATAC | GCTTCACTTTCTTACTTGGCG | [FAM] CCCTGGCAGCATCATCCACACATA [BH1] |
| BAX | GACATGTTTTCTGACGGCAAC | AAGTCCAATGTCCAGCCC | [FAM] CTGGCAAAGTAGAAAAGGGCGACAAC [BH1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaeva, K.V.; Chulpanova, D.S.; Zhuravleva, M.N.; Filin, I.Y.; Deviatiiarov, R.M.; Ballard-Reisch, A.C.; Rizvanov, A.A.; Solovyeva, V.V. Characteristics and Resistance to Cisplatin of Human Neuroblastoma Cells Co-Cultivated with Immune and Stromal Cells. Bioengineering 2022, 9, 655. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9110655
Kitaeva KV, Chulpanova DS, Zhuravleva MN, Filin IY, Deviatiiarov RM, Ballard-Reisch AC, Rizvanov AA, Solovyeva VV. Characteristics and Resistance to Cisplatin of Human Neuroblastoma Cells Co-Cultivated with Immune and Stromal Cells. Bioengineering. 2022; 9(11):655. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9110655
Chicago/Turabian StyleKitaeva, Kristina V., Daria S. Chulpanova, Margarita N. Zhuravleva, Ivan Yu. Filin, Ruslan M. Deviatiiarov, Alyssa C. Ballard-Reisch, Albert A. Rizvanov, and Valeriya V. Solovyeva. 2022. "Characteristics and Resistance to Cisplatin of Human Neuroblastoma Cells Co-Cultivated with Immune and Stromal Cells" Bioengineering 9, no. 11: 655. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9110655