Kinetics of Biodiesel Production from Microalgae Using Microbubble Interfacial Technology
Abstract
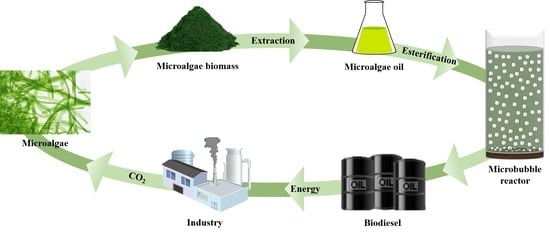
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Lipids Extraction from Spirulina Biomass
2.3. Pilot-Scale Experimental Setup for Biodiesel Production
2.4. Modeling and Experimental Design through Response Surface Methodology
2.5. Modeling through Gated Recurrent Unit (GRU)
2.6. Biodiesel Analysis
3. Results and Discussion
3.1. Effect of Different Parameters on Free Fatty Acid Conversion
3.1.1. Effect of Catalyst Loading and Molar Ratio on Free Fatty Acid Conversion
3.1.2. Effect of Reaction Time and Molar Ratio on Free Fatty Acid Conversion
3.1.3. Effect of Reaction Time and Catalyst Loading on Free Fatty Acid Conversion
3.2. Scale-Up of Microbubble Reactor
3.3. Reaction Kinetics of Biodiesel Conversion
3.4. Gated Recurrent Unit and Response Surface Methodology Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Words | Abbreviations |
| Activation energy | EA |
| Absolute percentage error | MAPE |
| Acid value | AV |
| Box–Behnken design | BBD |
| Concentration of MO | Cb |
| Enhancement factor | E |
| Free Fatty Acid | FFA |
| Fatty Acid Methyl Esters | FAMEs |
| Gas constant | R |
| Gated recurrent unit | GRU |
| Gas diffusion coefficient | Mg/l |
| Henry constant | H |
| Hatta number | Ha |
| Interfacial area | |
| Infinite enhancement factor | Ei |
| Liquid film coefficient | kbl |
| Mass of biodiesel (g) | W |
| Microalgae oil | MO |
| Methanol | MeOH |
| Molar volume of MO | vl |
| Molar volume of MeOH | vg |
| Mean absolute error | MAE |
| Normality of KOH | N |
| Partial pressure of MeOH | pg |
| Pressure of MeOH (bar) | PA |
| Pre-exponential factor | A° |
| Rate constant | K |
| Rate of reaction | ra |
| Root mean square error | RMSE |
| Response surface methodology | RSM |
| Volume of KOH used for titration (mL) | FA |
| Volume of KOH used for blank titration (mL) | FB |
References
- Malekghasemi, S.; Kariminia, H.-R.; Plechkova, N.K.; Ward, V.C. Direct transesterification of wet microalgae to biodiesel using phosphonium carboxylate ionic liquid catalysts. Biomass Bioenergy 2021, 150, 106126. [Google Scholar] [CrossRef]
- Javed, F.; Rehman, F.; Khan, A.U.; Fazal, T.; Hafeez, A.; Rashid, N. Real textile industrial wastewater treatment and biodiesel production using microalgae. Biomass Bioenergy 2022, 165, 106559. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef] [Green Version]
- Soccol, C.R.; Neto, C.J.D.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; de Souza Vandenberghe, L.P. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y.-T. Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Faisal, A.; Javed, F.; Hassan, M.; Gorji, M.; Akram, S.; Rashid, N.; Rehman, F. Experimental and mathematical nonlinear rheological characterization of chicken fat oil-a sustainable feedstock for biodiesel. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Shuit, S.-H.; Ong, H.-C. Utilisation of biomass wastes based activated carbon supported heterogeneous acid catalyst for biodiesel production. Renew. Energy 2020, 158, 91–102. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, Y.; Sharma, A. Optimization of biodiesel synthesis from Jojoba oil via supercritical methanol: A response surface methodology approach coupled with genetic algorithm. Biomass Bioenergy 2022, 156, 106332. [Google Scholar] [CrossRef]
- Meher, L.C.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Kokoo, R. Esterification for biodiesel production with a phantom catalyst: Bubble mediated reactive distillation. Appl. Energy 2018, 221, 28–40. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chang, J.-S.; Lee, D.-J. Recent insights into continuous-flow biodiesel production via catalytic and non-catalytic transesterification processes. Appl. Energy 2017, 185, 376–409. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Wu, Y.; Chen, J.; Li, S.; Ye, Y.; Wang, D.; Zheng, Z. Optimization of key parameters using RSM for improving the production of the green biodiesel from FAME by hydrotreatment over Pt/SAPO-11. Biomass Bioenergy 2022, 158, 106379. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Yang, X.-X. Biodiesel production from high acid value oils with a highly active and stable bifunctional magnetic acid. Appl. Energy 2017, 204, 702–714. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Narkhede, N.; Singh, S.; Patel, A. Recent progress on supported polyoxometalates for biodiesel synthesis via esterification and transesterification. Green Chem. 2015, 17, 89–107. [Google Scholar] [CrossRef]
- Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, R.; Akram, S.; Rashid, N.; Zimmerman, W.B.; Rehman, F. Conversion of Poultry-Fat Waste to a Sustainable Feedstock for Biodiesel Production via Microbubble Injection of Reagent Vapor. J. Clean. Prod. 2021, 311, 127525. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Ahmad, N.; Javed, F.; Awan, J.A.; Ali, S.; Fazal, T.; Hafeez, A.; Aslam, R.; Rashid, N.; Rehman, M.S.U.; Zimmerman, W.B. Biodiesel production intensification through microbubble mediated esterification. Fuel 2019, 253, 25–31. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Tesar, V.; Butler, S.; Bandulasena, H.C. Microbubble generation. Recent Pat. Eng. 2008, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, W.B.; Al-Mashhadani, M.K.; Bandulasena, H.H. Evaporation dynamics of microbubbles. Chem. Eng. Sci. 2013, 101, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Shamair, Z.; Ali, S.; Ahmad, N.; Hafeez, A.; Fazal, T.; Rehman, M.S.U.; Zimmerman, W.B.; Rehman, F. “Pushing and pulling” the equilibrium through bubble mediated reactive separation for ethyl acetate production. React. Chem. Eng. 2019, 4, 705–714. [Google Scholar] [CrossRef]
- Javed, F.; Rizwan, M.; Asif, M.; Ali, S.; Aslam, R.; Akram, M.S.; Zimmerman, W.B.; Rehman, F. Intensification of Biodiesel Processing from Waste Cooking Oil, Exploiting Cooperative Microbubble and Bifunctional Metallic Heterogeneous Catalysis. Bioengineering 2022, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Hariram, V.; Bose, A.; Seralathan, S. Dataset on optimized biodiesel production from seeds of Vitis vinifera using ANN, RSM and ANFIS. Data Brief 2019, 25, 104298. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, N.; Muthukumaran, C.; Sharmila, G. A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. J. King Saud Univ.-Eng. Sci. 2020, 34, 198–208. [Google Scholar] [CrossRef]
- Park, J.-Y.; Nam, B.; Choi, S.-A.; Oh, Y.-K.; Lee, J.-S. Effects of anionic surfactant on extraction of free fatty acid from Chlorella vulgaris. Bioresour. Technol. 2014, 166, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Jiao, W.; Yu, L.; Feng, Z.; Guo, L.; Wang, Y.; Liu, Y. Optimization of nitrobenzene wastewater treatment with O3/H2O2 in a rotating packed bed using response surface methodology. Desalin. Water Treat. 2016, 57, 19996–20004. [Google Scholar] [CrossRef]
- Ruhul, A.; Kalam, M.; Masjuki, H.; Fattah, I.R.; Reham, S.; Rashed, M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, N.; Venayagamoorthy, G.K.; Wunsch, D.C. Time series prediction with recurrent neural networks using a hybrid PSO-EA algorithm. In Proceedings of the 2004 IEEE International Joint Conference on Neural Networks (IEEE Cat. No.04CH37541), Budapest, Hungary, 25–29 July 2004; Volume 1642, pp. 1647–1652. [Google Scholar]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Cho, K.; Merrienboer, B.v.; Gülçehre, Ç.; Bahdanau, D.; Bougares, F.; Schwenk, H.; Bengio, Y. Learning Phrase Representations Using RNN Encoder Decoder for Statistical Machine Translation. In Proceedings of the EMNLP, Doha, Qatar, 25–29 October 2014. [Google Scholar]
- Saif-ul-Allah, M.W.; Qyyum, M.A.; Ul-Haq, N.; Salman, C.A.; Ahmed, F. Gated Recurrent Unit Coupled with Projection to Model Plane Imputation for the PM2.5 Prediction for Guangzhou City, China. Front. Environ. Sci. 2022, 9, 753. [Google Scholar] [CrossRef]
- Marchetti, J.; Errazu, A. Comparison of different heterogeneous catalysts and different alcohols for the esterification reaction of oleic acid. Fuel 2008, 87, 3477–3480. [Google Scholar] [CrossRef]
- Yin, P.; Chen, L.; Wang, Z.; Qu, R.; Liu, X.; Xu, Q.; Ren, S. Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418. Fuel 2012, 102, 499–505. [Google Scholar] [CrossRef]
- Rehman, F.; Medley, G.J.; Bandulasena, H.; Zimmerman, W.B. Fluidic oscillator-mediated microbubble generation to provide cost effective mass transfer and mixing efficiency to the wastewater treatment plants. Environ. Res. 2015, 137, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.D.; Turley, M.; Robinson, R.; Zimmerman, W.B. Hot microbubble injection in thin liquid film layers for ammonia separation from ammonia rich-wastewater. Chem. Eng. Process.-Process Intensif. 2021, 180, 108693. [Google Scholar] [CrossRef]
- Tashtoush, G.M.; Al-Widyan, M.I.; Al-Jarrah, M.M. Experimental study on evaluation and optimization of conversion of waste animal fat into biodiesel. Energy Convers. Manag. 2004, 45, 2697–2711. [Google Scholar] [CrossRef]
- Al-mashhadani, M.K.; Wilkinson, S.J.; Zimmerman, W.B. Laboratory preparation of simulated sludge for anaerobic digestion experimentation. J. Eng. 2015, 21, 131–145. [Google Scholar]
- Rees-Zimmerman, C.R.; Chaffin, S.T. Modelling the effect of bioreactor height on stripping fermentation products from the engineered bacterium Geobacillus thermoglucosidasius. Biochem. Eng. J. 2021, 176, 108195. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Kierzkowska-Pawlak, H. Determination of kinetics in gas-liquid reaction systems. An overview. Ecol. Chem. Eng. S 2012, 19, 175–196. [Google Scholar] [CrossRef]
- Díaz, M.; Vega, A.; Coca, J. Correlation for the estimation of gas-liquid diffusivity. Chem. Eng. Commun. 1987, 52, 271–281. [Google Scholar] [CrossRef]
- Kimweri, H.T.H. Enhancement of Gas-Liquid Mass Transfer in Hydrometallurgical Leaching Systems. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2001. [Google Scholar]
- Roman, F.F.; Ribeiro, A.E.; Queiroz, A.; Lenzi, G.G.; Chaves, E.S.; Brito, P. Optimization and kinetic study of biodiesel production through esterification of oleic acid applying ionic liquids as catalysts. Fuel 2019, 239, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Sharma, M. Kinetics of acid base catalyzed transesterification of Jatropha curcas oil. Bioresour. Technol. 2010, 101, 7701–7706. [Google Scholar] [CrossRef]
- Song, E.-S.; Lim, J.-W.; Lee, H.-S.; Lee, Y.-W. Transesterification of RBD palm oil using supercritical methanol. J. Supercrit. Fluids 2008, 44, 356–363. [Google Scholar] [CrossRef]
- Ahmad, A.; Yasin, N.M.; Derek, C.; Lim, J. Kinetic studies and thermodynamics of oil extraction and transesterification of Chlorella sp. for biodiesel production. Environ. Technol. 2014, 35, 891–897. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.; Dastidar, M. Kinetic and thermodynamic studies on biodiesel production from Spirulina platensis algae biomass using single stage extraction–transesterification process. Fuel 2014, 135, 228–234. [Google Scholar] [CrossRef]










| Parameters | Units | Value |
|---|---|---|
| FFA content | % | 32.5 ± 2 |
| Density (25 °C) | Kg m3 | 920 ± 5 |
| Kinematic viscosity (40 °C) | mm2 s−1 | 30.06 ± 3 |
| MO composition | ||
| Mystic acid (C14:0) | % | 1.90 ± 0.5 |
| Palmitic acid (C16:0) | % | 35.67 ± 3 |
| Palmitoleic acid (C16:1) | % | 6.11 ± 2 |
| Linoleic acid (C18:2) | % | 48.55 ± 2 |
| Linolenic acid (C18:3) | % | 2.17 ± 0.5 |
| Stearic acid (C18:0) | % | 5.60 ± 2 |
| Hyperparameters | Bounds | Set Values |
|---|---|---|
| Number of hidden units | Positive integers | 50 |
| Gradient threshold | 0–1 | 0.1 |
| Initial learning rate | 0–1 | 0.01 |
| Learn rate drop factor | 0–1 | 0.2 |
| Learn rate drop period | Positive integers | 100 |
| Training Epochs | Positive integers | 150 |
| Feedstock | Catalyst | Reaction Time (min) | Conversion (%) | Reference |
|---|---|---|---|---|
| Conventional method | H2SO4 | 120 | 78 | [40] |
| Microbubble Technology | H2SO4 | 30 | 98 | [20] |
| Microbubble Technology | p-TSA | 30 | 97 | [20] |
| Microbubble Technology | p-TSA | 30 | 89.90 | [18] |
| Microbubble Technology | Sr/ZrO2 | 20 | 85 | [24] |
| Microbubble Technology (semi pilot-scale) | p-TSA | 60 | 99.45 ± 1.3 | This study |
| Feedstock | Method | Scale of Experiments | Catalyst | EA (kJ mol−1) | Reference |
|---|---|---|---|---|---|
| Jatropha | Conventional method | Lab-scale | 1% H2SO4 and 1% NaOH | 87.808 | [48] |
| Microalgae | Supercritical method | Lab-scale | No catalyst | 105 | [49] |
| Chlorella | Conventional method | Lab-scale | HCl | 38.892 | [50] |
| Spirulina platensis | Single stage extraction–transesterification process | Lab-scale | H2SO4 | 14.518 | [51] |
| Oleic acid | Microbubble technology | Lab-scale | 7% H2SO4 | 26.37 | [20] |
| Chicken fat oil | Microbubble technology | Lab-scale | 7% PTSA | 24.9 | [18] |
| Spirulina | Microbubble technology | Semi pilot scale | 3.3% PTSA | 10.01 ± 0.3 | This study |
| Criteria | Conversion % Prediction Performance | |
|---|---|---|
| RSM Model | GRU Model | |
| R2 | 0.9844 | 0.9999 |
| MAPE | 0.0465 | 0.00083 |
| RMSE | 3.0832 | 0.0515 |
| MAE | 2.6847 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, F.; Saif-ul-Allah, M.W.; Ahmed, F.; Rashid, N.; Hussain, A.; Zimmerman, W.B.; Rehman, F. Kinetics of Biodiesel Production from Microalgae Using Microbubble Interfacial Technology. Bioengineering 2022, 9, 739. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9120739
Javed F, Saif-ul-Allah MW, Ahmed F, Rashid N, Hussain A, Zimmerman WB, Rehman F. Kinetics of Biodiesel Production from Microalgae Using Microbubble Interfacial Technology. Bioengineering. 2022; 9(12):739. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9120739
Chicago/Turabian StyleJaved, Fahed, Muhammad Waqas Saif-ul-Allah, Faisal Ahmed, Naim Rashid, Arif Hussain, William B. Zimmerman, and Fahad Rehman. 2022. "Kinetics of Biodiesel Production from Microalgae Using Microbubble Interfacial Technology" Bioengineering 9, no. 12: 739. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9120739