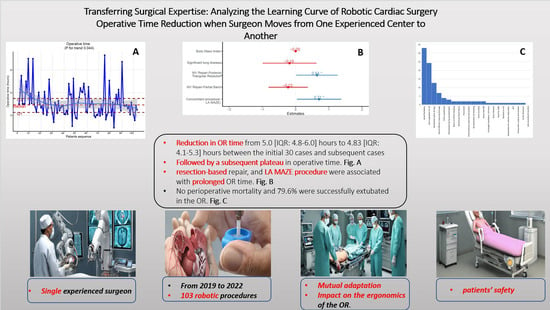
Transferring Surgical Expertise: Analyzing the Learning Curve of Robotic Cardiac Surgery Operative Time Reduction When Surgeon Moves from One Experienced Center to Another
Abstract
:1. Introduction
2. Patients and Methods
2.1. Inclusion, and Exclusion Criteria
2.2. Study Type and Data Retrieval
2.3. End Points and Outcomes Definition
2.4. Data Statistical Analysis
3. Results
3.1. Patients’ Demographics
3.2. Detailed Mitral Pathology
3.3. Details of the Used Repair Methods
3.4. Assessment of Technical Proficiency
3.5. Assessment of Operative Success
3.6. Assessment of Patient Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Members, A.T.F.; Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M. Guidelines on the management of valvular heart disease (version 2012) The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar]
- Bolling, S.F.; Li, S.; O’Brien, S.M.; Brennan, J.M.; Prager, R.L.; Gammie, J.S. Predictors of mitral valve repair: Clinical and surgeon factors. Ann. Thorac. Surg. 2010, 90, 1904–1912. [Google Scholar] [CrossRef]
- Gammie, J.S.; O’Brien, S.M.; Griffith, B.P.; Ferguson, T.B.; Peterson, E.D. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation 2007, 115, 881–887. [Google Scholar] [CrossRef]
- LaPar, D.J.; Ailawadi, G.; Isbell, J.M.; Crosby, I.K.; Kern, J.A.; Rich, J.B.; Speir, A.M.; Kron, I.L.; Investigators for the Virginia Cardiac Surgery Quality Initiative. Mitral valve repair rates correlate with surgeon and institutional experience. J. Thorac. Cardiovasc. Surg. 2014, 148, 995–1004. [Google Scholar] [CrossRef]
- Kilic, A.; Shah, A.S.; Conte, J.V.; Baumgartner, W.A.; Yuh, D.D. Operative outcomes in mitral valve surgery: Combined effect of surgeon and hospital volume in a population-based analysis. J. Thorac. Cardiovasc. Surg. 2013, 146, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Chikwe, J.; Toyoda, N.; Anyanwu, A.C.; Itagaki, S.; Egorova, N.N.; Boateng, P.; El-Eshmawi, A.; Adams, D.H. Relation of mitral valve surgery volume to repair rate, durability, and survival. J. Am. Coll. Cardiol. 2017, 69, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Loulmet, D.; Aupecle, B.; Kieffer, J.; Tournay, D.; Guibourt, P.; Fiemeyer, A.; Méléard, D.; Richomme, P.; Cardon, C. Computer assisted open heart surgery. First case operated on with success. Comptes Rendus L’academie Des. Sci. Ser. III Sci. Vie 1998, 321, 437–442. [Google Scholar]
- Chitwood, W.R.; Nifong, L.W.; Elbeery, J.E.; Chapman, W.H.; Albrecht, R.; Kim, V.; Young, J.A. Robotic mitral valve repair: Trapezoidal resection and prosthetic annuloplasty with the da Vinci surgical system. J. Thorac. Cardiovasc. Surg. 2000, 120, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.; Koprivanac, M.; Kelava, M.; Mick, S.L.; Gillinov, A.M.; Rajeswaran, J.; Brzezinski, A.; Blackstone, E.H.; Mihaljevic, T. Robotic mitral valve repair: The learning curve. Innovations 2017, 12, 390–397. [Google Scholar] [PubMed]
- Seo, Y.J.; Sanaiha, Y.; Bailey, K.L.; Aguayo, E.; Chao, A.; Shemin, R.J.; Benharash, P. Outcomes and resource utilization in robotic mitral valve repair: Beyond the learning curve. J. Surg. Res. 2019, 235, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Yaffee, D.W.; Loulmet, D.F.; Kelly, L.A.; Ward, A.F.; Ursomanno, P.A.; Rabinovich, A.E.; Neuburger, P.J.; Krishnan, S.; Hill, F.T.; Grossi, E.A. Can the learning curve of totally endoscopic robotic mitral valve repair be short-circuited? Innovations 2014, 9, 43–48. [Google Scholar] [PubMed]
- Ruppert, D.; Wand, M.P.; Carroll, R.J. Semiparametric Regression; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Singhal, S.; Kayyali, B.; Levin, R.; Greenberg, Z. The Next Wave of Healthcare Innovation: The Evolution of Ecosystems; McKinsey & Company: Chicago, IL, USA, 2020. [Google Scholar]
- Laihonen, H. Knowledge structures of a health ecosystem. J. Health Organ. Manag. 2012, 26, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Scarponi, D.; Cangini, G.; Pasini, A.; La Scola, C.; Mencarelli, F.; Bertulli, C.; Amabile, D.; Busutti, M.; La Manna, G.; Pession, A. The process of transition from pediatric to adult healthcare services for nephrological patients: Recommendations vs. reality—A single center experience. Front. Pediatr. 2022, 10, 954641. [Google Scholar] [CrossRef] [PubMed]
- Kjellström, S.; Avby, G.; Areskoug-Josefsson, K.; Andersson Gäre, B.; Andersson Bäck, M. Work motivation among healthcare professionals: A study of well-functioning primary healthcare centers in Sweden. J. Health Organ. Manag. 2017, 31, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Behruzi, R.; Klam, S.; Dehertog, M.; Jimenez, V.; Hatem, M. Understanding factors affecting collaboration between midwives and other health care professionals in a birth center and its affiliated Quebec hospital: A case study. BMC Pregnancy Childbirth 2017, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Arrow, H.; McGrath, J.E.; Staw, B.; Cummings, L. Membership dynamics in groups at work: A theoretical framework. Res. Organ. Behav. 1995, 17, 373. [Google Scholar]
- Levi, D.; Askay, D.A. Group Dynamics for Teams; Sage Publications: Thousand Oaks, CA, USA, 2020. [Google Scholar]
- Wong, S.W.; Smith, R.; Crowe, P. Optimizing the operating theatre environment. ANZ J. Surg. 2010, 80, 917–924. [Google Scholar] [CrossRef]
- Arora, S.; Sevdalis, N.; Nestel, D.; Woloshynowych, M.; Darzi, A.; Kneebone, R. The impact of stress on surgical performance: A systematic review of the literature. Surgery 2010, 147, 318–330.e6. [Google Scholar] [CrossRef]
- Helmreich, R.L.; Schaefer, H.-G. Team performance in the operating room. Hum. Error Med. 1994, 225, 253. [Google Scholar]
- Adams, D.; Swain, J.; Accola, K. Standards and Best Practices for Mitral Valve Repair Reference Centers. Mitral Found. 2020. [Google Scholar]
- Gammie, J.S.; Chikwe, J.; Badhwar, V.; Thibault, D.P.; Vemulapalli, S.; Thourani, V.H.; Gillinov, M.; Adams, D.H.; Rankin, J.S.; Ghoreishi, M. Isolated mitral valve surgery: The society of thoracic surgeons adult cardiac surgery database analysis. Ann. Thorac. Surg. 2018, 106, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Gillinov, A.M.; Blackstone, E.H.; Alaulaqi, A.; Sabik, J.F., III; Mihaljevic, T.; Svensson, L.G.; Houghtaling, P.L.; Salemi, A.; Johnston, D.R.; Lytle, B.W. Outcomes after repair of the anterior mitral leaflet for degenerative disease. Ann. Thorac. Surg. 2008, 86, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Cohen, J.E.; Howard, J.L.; Edwards, B.B.; Acker, A.L.; Hiesinger, W.; MacArthur, J.W., Jr.; Atluri, P.; Woo, Y.J. A “repair-all” strategy for degenerative mitral valve disease safely minimizes unnecessary replacement. Ann. Thorac. Surg. 2015, 99, 1983–1991. [Google Scholar] [CrossRef]
- Chikwe, J.; Goldstone, A.B.; Passage, J.; Anyanwu, A.C.; Seeburger, J.; Castillo, J.G.; Filsoufi, F.; Mohr, F.W.; Adams, D.H. A propensity score-adjusted retrospective comparison of early and mid-term results of mitral valve repair versus replacement in octogenarians. Eur. Heart J. 2011, 32, 618–626. [Google Scholar] [CrossRef]



| Overall | After | First 30 Cases | p | |
| N | 103 | 73 | 30 | |
| Age (years (median [IQR]) | 63.00 [56.50, 69.00] | 62.00 [53.00, 68.00] | 65.50 [58.25, 69.00] | 0.235 |
| Gender (Males %) | 70 (68.0) | 52 (71.2) | 18 (60.0) | 0.38 |
| Diagnosis | ||||
| 93 (90.3) | 67 (91.8) | 26 (86.7) | 0.667 |
| 2 (1.9) | 0 (0.0) | 2 (6.7) | 0.149 |
| 7 (6.8) | 4 (5.5) | 3 (10.0) | 0.691 |
| 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| 9 (8.7) | 5 (6.8) | 4 (13.3) | 0.5 |
| 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| 3 (2.9) | 0 (0.0) | 3 (10.0) | 0.036 |
| 6 (5.8) | 3 (4.1) | 3 (10.0) | 0.486 |
| Body Mass Index (median [IQR]) | 23.87 [22.02, 26.49] | 24.34 [22.07, 26.18] | 23.77 [21.94, 26.83] | 0.925 |
| Prior HTN (%) | 47 (45.6) | 32 (43.8) | 15 (50.0) | 0.724 |
| Prior DM (%) | 3 (2.9) | 3 (4.1) | 0 (0.0) | 0.63 |
| Prior HLD (%) | 41 (39.8) | 26 (35.6) | 15 (50.0) | 0.257 |
| Prior CAD (%) | 9 (8.7) | 7 (9.6) | 2 (6.7) | 0.926 |
| Prior Peripheral artery disease (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Prior Atrial Fibrillation (%) | 21 (20.4) | 12 (16.4) | 9 (30.0) | 0.199 |
| Prior Atrial Flutter (%) | 3 (2.9) | 1 (1.4) | 2 (6.7) | 0.419 |
| Prior MI (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Prior Significant lung disease (%) | 9 (8.7) | 6 (8.2) | 3 (10.0) | 1 |
| Prior Liver disease (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Prior CVA (%) | 3 (2.9) | 2 (2.7) | 1 (3.3) | 1 |
| Prior Smoking (%) | 27 (26.2) | 19 (26.0) | 8 (26.7) | 1 |
| NYHA class (%) | ||||
| 20 (19.4) | 16 (21.9) | 4 (13.3) | 0.507 |
| 57 (55.3) | 38 (52.1) | 19 (63.3) | |
| 26 (25.2) | 19 (26.0) | 7 (23.3) | |
| Ejection fraction, ___% (median [IQR]) | 62.00 [55.50, 65.00] | 61.00 [58.00, 65.00] | 65.00 [54.00, 67.00] | 0.881 |
| AI (%) | ||||
| 72 (69.9) | 49 (67.1) | 23 (76.7) | 0.428 |
| 29 (28.2) | 23 (31.5) | 6 (20.0) | |
| 2 (1.9) | 1 (1.4) | 1 (3.3) | |
| MR (%) | ||||
| 8 (7.8) | 5 (6.8) | 3 (10.0) | 0.294 |
| 1 (1.0) | 0 (0.0) | 1 (3.3) | |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | |
| 17 (16.5) | 10 (13.7) | 7 (23.3) | |
| 76 (73.8) | 57 (78.1) | 19 (63.3) | |
| TR (%) | ||||
| 50 (48.5) | 34 (46.6) | 16 (53.3) | 0.224 |
| 48 (46.6) | 35 (47.9) | 13 (43.3) | |
| 4 (3.9) | 4 (5.5) | 0 (0.0) | |
| 1 (1.0) | 0 (0.0) | 1 (3.3) | |
| Etiology of MV disease | ||||
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 91 (88.3) | 65 (89.0) | 26 (86.7) | 0.997 |
| 2 (1.9) | 2 (2.7) | 0 (0.0) | 0.897 |
| 15 (14.6) | 12 (16.4) | 3 (10.0) | 0.593 |
| 2 (1.9) | 2 (2.7) | 0 (0.0) | 0.897 |
| MV lesions | ||||
| 66 (64.1) | 48 (65.8) | 18 (60.0) | 0.744 |
| 9 (8.7) | 4 (5.5) | 5 (16.7) | 0.149 |
| 15 (14.6) | 11 (15.1) | 4 (13.3) | 1 |
| 72 (69.9) | 50 (68.5) | 22 (73.3) | 0.802 |
| 30 (29.1) | 27 (37.0) | 3 (10.0) | 0.012 |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 5 (4.9) | 4 (5.5) | 1 (3.3) | 1 |
| 13 (12.6) | 11 (15.1) | 2 (6.7) | 0.401 |
| Carpentier MR Classification (%) | ||||
| 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| 93 (90.3) | 67 (91.8) | 26 (86.7) | 0.667 |
| 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| 6 (5.8) | 3 (4.1) | 3 (10.0) | 0.486 |
| Overall | After | First 30 Cases | p | |
| Operative details and outcomes | 103 | 73 | 30 | |
| Operative time (mean (SD)) | 5.10 (1.21) | 4.93 (1.15) | 5.51 (1.28) | 0.026 |
| Operative time (median [IQR]) | 4.92 [4.26, 5.48] | 4.83 [4.10, 5.27] | 5.00 [4.76, 6.00] | 0.01 |
| No. of pump runs (%) | ||||
| 94 (91.3) | 66 (90.4) | 28 (93.3) | 0.656 |
| 7 (6.8) | 5 (6.8) | 2 (6.7) | |
| 2 (1.9) | 2 (2.7) | 0 (0.0) | |
| Operations performed: | ||||
| 93 (90.3) | 67 (91.8) | 26 (86.7) | 0.667 |
| 4 (3.9) | 2 (2.7) | 2 (6.7) | 0.707 |
| 3 (2.9) | 2 (2.7) | 1 (3.3) | 1 |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 15 (14.6) | 9 (12.3) | 6 (20.0) | 0.487 |
| 6 (5.8) | 3 (4.1) | 3 (10.0) | 0.486 |
| 7 (6.8) | 4 (5.5) | 3 (10.0) | 0.691 |
| 7 (6.8) | 4 (5.5) | 3 (10.0) | 0.691 |
| 6 (5.8) | 3 (4.1) | 3 (10.0) | 0.486 |
| If MV Repair: | ||||
| 17 (16.5) | 12 (16.4) | 5 (16.7) | 1 |
| 9 (8.7) | 8 (11.0) | 1 (3.3) | 0.389 |
| 9 (8.7) | 9 (12.3) | 0 (0.0) | 0.103 |
| 60 (58.3) | 43 (58.9) | 17 (56.7) | 1 |
| 8 (7.8) | 5 (6.8) | 3 (10.0) | 0.891 |
| 81 (78.6) | 58 (79.5) | 23 (76.7) | 0.961 |
| 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 8 (7.8) | 7 (9.6) | 1 (3.3) | 0.501 |
| 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| 10 (9.7) | 7 (9.6) | 3 (10.0) | 1 |
| 33 (32.0) | 23 (31.5) | 10 (33.3) | 1 |
| 18 (17.5) | 8 (11.0) | 10 (33.3) | 0.015 |
| Cardiopulmonary Bypass Time (median [IQR]) | 145.00 [130.00, 174.50] | 144.00 [128.50, 173.00] | 144.00 [128.50, 173.00] | 0.255 |
| Aortic Cross-clamp time (median [IQR]) | 82.00 [72.00, 95.75] | 83.00 [70.75, 94.25] | 81.00 [74.25, 96.00] | 0.719 |
| Did the patient receive blood products in the OR? (%) | 23 (22.5) | 17 (23.6) | 6 (20.0) | 0.891 |
| MR grade at the end of the case (%) | ||||
| 65 (63.7) | 51 (69.9) | 14 (48.3) | 0.123 |
| 32 (31.4) | 19 (26.0) | 13 (44.8) | |
| 5 (4.9) | 3 (4.1) | 2 (6.9) | |
| Extubation in in the OR (%) | 82 (79.6) | 56 (76.7) | 26 (86.7) | 0.384 |
| Conversion to open procedure (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Postoperative blood product (%) | 16 (15.5) | 12 (16.4) | 4 (13.3) | 0.924 |
| Postoperative complications | ||||
| Return to OR for bleeding (%) | 2 (1.9) | 2 (2.7) | 0 (0.0) | 0.897 |
| Prolonged Ventilation >24 h (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Reintubated during hospitalization (%) | 3 (2.9) | 1 (1.4) | 2 (6.7) | 0.419 |
| New/Acute Renal Failure (%) | 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| Groin Infection (%) | 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| Groin Lymphocele (%) | 4 (3.9) | 2 (2.7) | 2 (6.7) | 0.707 |
| Need for IABP (%) | 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| Readmit to ICU (%) | 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| Reoperation for valvular dysfunction within 30 days (%) | 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| Pneumonia (%) | 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| Pleural Effusion requiring drainage (%) | 7 (6.8) | 6 (8.2) | 1 (3.3) | 0.642 |
| DVT (%) | 1 (1.0) | 0 (0.0) | 1 (3.3) | 0.644 |
| Pneumothorax requiring intervention (%) | 2 (1.9) | 1 (1.4) | 1 (3.3) | 1 |
| Tamponade, surgical intervention (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Aortic Dissection (%) | 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Atrial Fibrillation (%) | 34 (33.0) | 21 (28.8) | 13 (43.3) | 0.231 |
| Uneventful post operative course (%) | 36 (35.0) | 25 (34.2) | 11 (36.7) | 0.995 |
| If re-exploration for bleeding: | ||||
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| 1 (1.0) | 1 (1.4) | 0 (0.0) | 1 |
| Anti-coagulation required in AF (%) | 25 (24.3) | 15 (20.5) | 10 (33.3) | 0.262 |
| Did the patient survive 30 day or discharge whichever is longer? (%) | 101 (99.0) | 72 (100.0) | 29 (96.7) | 0.65 |
| Readmitted within 30 days? (%) | 13 (12.7) | 9 (12.5) | 4 (13.3) | 1 |
| ICU stay (days) (median [IQR]) | 2.00 [2.00, 3.00] | 2.00 [2.00, 3.00] | 2.00 [2.00, 4.00] | 0.293 |
| Last follow-up status (Alive (%)) | 103 (100.0) | 73 (100.0) | 30 (100.0) | NA |
| MR degree at last follow up (%) | ||||
| 66 (64.1) | 45 (61.6) | 21 (70.0) | 0.082 |
| 12 (11.7) | 7 (9.6) | 5 (16.7) | |
| 1 (1.0) | 0 (0.0) | 1 (3.3) | |
| 2 (1.9) | 1 (1.4) | 1 (3.3) | |
| 22 (21.4) | 20 (27.4) | 2 (6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairallah, S.M.; Rahouma, M.; Mick, S.L. Transferring Surgical Expertise: Analyzing the Learning Curve of Robotic Cardiac Surgery Operative Time Reduction When Surgeon Moves from One Experienced Center to Another. J. Cardiovasc. Dev. Dis. 2024, 11, 81. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd11030081
Khairallah SM, Rahouma M, Mick SL. Transferring Surgical Expertise: Analyzing the Learning Curve of Robotic Cardiac Surgery Operative Time Reduction When Surgeon Moves from One Experienced Center to Another. Journal of Cardiovascular Development and Disease. 2024; 11(3):81. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd11030081
Chicago/Turabian StyleKhairallah, Sherif M., Mohamed Rahouma, and Stephanie L. Mick. 2024. "Transferring Surgical Expertise: Analyzing the Learning Curve of Robotic Cardiac Surgery Operative Time Reduction When Surgeon Moves from One Experienced Center to Another" Journal of Cardiovascular Development and Disease 11, no. 3: 81. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd11030081