Maternal Outcomes in Women with Peripartum Cardiomyopathy versus Age and Race-Matched Peers in an Urban US Community
Abstract
:1. Introduction
2. Materials and Methods
Demographic and Clinical Variables
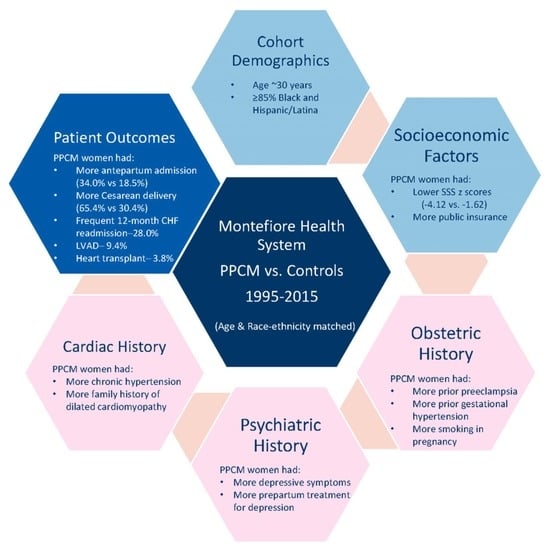
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosures
Abbreviations
| PPCM | Peripartum cardiomyopathy |
| LVEF | Left ventricular ejection fraction |
References
- MacDorman, M.F.; Declercq, E.; Thoma, M.E. Trends in Maternal Mortality by Sociodemographic Characteristics and Cause of Death in 27 States and the District of Columbia. Obs. Gynecol. 2017, 129, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, R.M.; Gaither, K.; Nasser, S.A.; Albert, M.A.; Ferdinand, K.C.; Njoroge, J.C.; Parapid, B.; Hayes, S.N.; Pegus, C.; Sogade, B.; et al. Working Agenda for Black Mothers: A Position Paper from the Association of Black Cardiologists on Solutions to Improving Black Maternal Health. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007643. [Google Scholar] [CrossRef]
- Elkayam, U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J. Am. Coll. Cardiol. 2011, 58, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.; Mebazaa, A.; Pieske, E.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, M.B.; Dias, J.K.; Luis, A.; Patel, R.; Thornton, J.; Reed, G.L. African-American women have a higher risk for developing peripartum cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Goland, S.; Modi, K.; Hatamizadeh, P.; Elkayam, U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J. Card. Fail. 2013, 19, 214–218. [Google Scholar] [CrossRef]
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 207–221. [Google Scholar] [CrossRef]
- Hameed, A.B.; Lawton, E.S.; McCain, C.L.; Morton, C.H.; Mitchell, C.; Main, E.K.; Foster, E. Pregnancy-related cardiovascular deaths in California: Beyond peripartum cardiomyopathy. Am. J. Obs. Gynecol. 2015, 213, 379. [Google Scholar] [CrossRef]
- Goli, R.; Li, J.; Brandimarto, J.; Levine, L.D.; Riis, V.; McAfee, Q.; DePalma, S.; Haghighi, A.; Seidman, J.G.; Seidman, C.; et al. Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation 2021, 143, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Elkayam, U. Peripartum Cardiomyopathy. Circulation 2016, 133, 1397–1409. [Google Scholar] [CrossRef]
- Bortnick, A.E.; Buchwald, C.L.v.; Hasani, A.; Liu, C.; Berkowitz, J.L.; Vega, S.; Mustehsan, M.H.; Wolfe, D.S.; Taub, C. Persistence of abnormal global longitudinal strain in women with peripartum cardiomyopathy. Echocardiography 2021, 38, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.C.; Barasa, A.; Basic, C.; Nyberg, N.; Schaufelberger, M. Increased arterial stiffness and reduced left ventricular long-axis function in patients recovered from peripartum cardiomyopathy. Clin. Physiol. Funct. Imaging 2021, 41, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U.; Tummala, P.P.; Rao, K.; Akhter, M.W.; Karaalp, L.S. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N. Engl. J. Med. 2001, 344, 1567–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez Roux, A.V.; Merkin, S.S.; Arnett, D.; Chambless, L.; Massing, M.; Nieto, F.J.; Sorlie, P.; Szklo, M.; Tyroler, H.A.; Watson, R.L. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001, 345, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, N.; Rendon, I.S.H.; Arany, Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 1715–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obs. Gynecol. 2019, 133, 1.
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Galli, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obs.. Gynecol. 2022, 226, S786–S803. [Google Scholar] [CrossRef] [PubMed]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef]
- Biteker, M. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N. Engl. J. Med. 2016, 374, 2601. [Google Scholar]
- Davis, M.B.; Jarvie, J.; Gambahaya, E.; Lindenfeld, J.; Kao, D. Risk Prediction for Peripartum Cardiomyopathy in Delivering Mothers: A Validated Risk Model: PPCM Risk Prediction Model. J. Card. Fail. 2021, 27, 159–167. [Google Scholar] [CrossRef]
- Copperi, F.; Kim, J.D.; Diano, S. Melanocortin Signaling Connecting Systemic Metabolism with Mood Disorders. Biol. Psychiatry. 2022, 91, 879–887. [Google Scholar] [CrossRef]
- Child, S.T.; Ruppel, E.H.; Albert, M.A.; Lawton, L. Network Support and Negative Life Events Associated With Chronic Cardiometabolic Disease Outcomes. Am. J. Prev. Med. 2022, 62, e21–e28. [Google Scholar] [CrossRef]
- Rosman, L.; Salmoriago-Blotcher, E.; Cahill, J.; Wuensch, K.L.; Sears, S.F. Depression and health behaviors in women with Peripartum Cardiomyopathy. Heart Lung 2017, 46, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Goland, S.; Bitar, F.; Modi, K.; Safirstein, J.; Ro, A.; Mirocha, J.; Khatri, N.; Elkayam, U. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J. Card. Fail. 2011, 17, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.; Siedlecki, C.A.; Jhun, C.-S.; Weiss, W.J.; Manning, K.; Deutsch, S.; Pierce, W. Acquired Von Willebrand Syndrome and Blood Pump Design. Artif. Organs 2018, 42, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Winchester, M.-L.; Gorman, K.; Parrott, J.; Paeeish, M. Left ventricular assist device in pregnancy: Case report and review of the literature. J. Obstet. Gynaecol. Res. 2021, 47, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group Practice Bulletin No. 186: Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Obs. Gynecol. 2017, 130, e251–e269.
- Luthra, K.; Avula, S.; Raju, M.; Gangu, K. Risk factors and outcomes associated with Left Ventricular Thrombus in patients with Peripartum Cardiomyopathy: An insight from National Inpatient Sample Database. Am. J. Prev. Cardiol. 2022, 9, 100313. [Google Scholar] [CrossRef]
- Hilfiker-Kleiner, D.; Haghikia, A.; Berliner, D.; Vogel-Claussen, J.; Schwab, J.; Franke, A.; Schwarzkopf, M.; Ehlermann, P.; Pfister, R.; Michels, G.; et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur. Heart 2017, 38, 2671–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, D.M.; Elkayam, U.; Alharethi, R.; Damp, J.; Hsich, E.; Ewald, G.; Modi, K.; Alexis, J.D.; Ramani, G.V.; Semigran, M.J.; et al. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J. Am. Coll. Cardiol. 2015, 66, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Covariate | PPCM (n = 53) | Controls (n = 92) | p Value |
|---|---|---|---|
| Age, y | 32 ± 7 | 30 ± 7 | 0.96 |
| Race n, % | 0.58 | ||
| Black/African American | 32, 60.4 | 51, 55.4 | |
| Hispanic/Latina | 15, 28.3 | 27, 29.3 | |
| Non-Hispanic White | 2, 3.8 | 6, 6.5 | |
| Other | 2, 3.8 | 5, 5.4 | |
| Southeast Asian | 1, 1.9 | 2, 2.2 | |
| Not reported | 1, 1.9 | 1, 1.1 | |
| Black/African American | 32, 60.4 | 51, 55.4 | |
| Country of origin outside US | 4, 7.6% | 11, 12.0% | 0.57 |
| English as preferred language | 46, 86.8 | 87, 94.6 | 0.10 |
| Summary Socioeconomic Score | −4.12 (−6.81, −2.13) | −1.62 (−4.20, −0.74) | <0.001 |
| Gravida n | 3 (2, 5) | 3 (2, 5) | 0.70 |
| Para n | 2 (1, 3) | 1 (0, 2) | 0.16 |
| Body mass index ≥30 n, % | 30, 60.0 | 28, 43.1 | 0.07 |
| Chronic hypertension n, % | 13, 24.5 | 8, 8.8 | 0.001 |
| Prior gestational hypertension n, % | 11, 20.8 | 5, 5.4 | 0.001 |
| Prior preeclampsia n, % | 9, 17.0 | 3, 3.3 | 0.001 |
| Asthma n, % | 9, 17.0 | 18, 19.6 | 0.70 |
| Prior history of PPCM n, % | 7, 13.2 | 0, 0.0 | 0.001 |
| Family history of dilated cardiomyopathy n, % | 3, 5.7 | 0, 0.0 | 0.04 |
| Chronic diabetes n, % | 3, 5.7 | 2, 2.2 | 0.27 |
| Lupus or rheumatoid arthritis n,% | 0, 0.0 | 3, 3.3 | 0.18 |
| Hypothyroidism n, % | 2, 3.8 | 3, 3.3 | 0.87 |
| Hyperthyroidism n,% | 1, 1.9 | 1, 1.0 | 0.69 |
| End-stage renal disease n, % | 1, 1.9 | 0, 0.0 | 0.001 |
| Smoking during pregnancy n, % | 8, 15.1 | 2, 2.2 | 0.001 |
| Alcohol during pregnancy n, % | 2, 3.8 | 0,0.0 | 0.001 |
| Insurance n, % | 0.001 | ||
| Public | 36, 67.9 | 27, 29.3 | |
| Commercial | 13, 24.5 | 40, 43.5 | |
| Uninsured | 2, 3.8 | 22, 23.9 | |
| Not reported | 2, 3.8 | 3, 3.3 |
| Covariate | PPCM (n = 53) | Controls (n = 92) | p Value |
|---|---|---|---|
| Prior psychiatric history n, % | 16, 30.2 | 20, 21.7 | 0.26 |
| Any active psychiatric diagnosis n, % | 15, 28.3 | 14, 15.2 | 0.06 |
| Depressive symptoms | 7, 13.2 | 0, 0.0 | 0.001 |
| Depressive symptoms diagnosed postpartum | 9, 17.0 | 3, 3.3 | 0.009 |
| Depressive symptoms treated with medication | 9, 17.0 | 4, 4.4 | 0.02 |
| Treated with psychiatric medication prepartum | 3, 5.7 | 0, 0.0 | 0.047 |
| Treated with psychiatric medication postpartum | 4, 7.6 | 3, 3.3 | 0.26 |
| Anxiety symptoms | 8, 15.1 | 5, 5.4 | 0.05 |
| In-Hospital Cardiac Medications n, % | |
|---|---|
| Beta blocker | 46, 86.8 |
| Diuretic | 43, 81.1 |
| ACEi or ARB | 39, 73.6 |
| Warfarin | 11, 20.8 |
| Aldosterone receptor antagonist | 12, 22.6 |
| Hydralazine and/or isosorbide mononitrate or dinitrate | 11, 20.8 |
| Digoxin | 11, 20.8 |
| Aspirin | 7, 13.2 |
| Inotrope | 5, 9.4 |
| Nitroglycerin | 3, 5.7 |
| Calcium channel blocker | 3, 5.7 |
| Bromocriptine | 2, 3.8 |
| Methyldopa | 1, 1.9 |
| Cardiac Outcomes | p Value | |
|---|---|---|
| LVEF at diagnosis, % LVEF at 12-month follow-up (n = 34) *, % | 29 ± 11 43 ± 14 | <0.0001 |
| Follow-up with CHF subspecialist n, % | 28, 52.8 | |
| 12-month CHF rehospitalization, n % | 13, 24.5 | |
| ICD n, % | 9, 16.9 | |
| Mechanical ventilation n, % | 7, 13.2 | |
| LVAD, n, % | 5, 9.4 | |
| Heart transplant, n, % | 2, 3.8 | |
| Intra-aortic balloon pump | 1, 1.9 | |
| In-hospital maternal death n, % | 0, 0.0 |
| Antepartum Maternal Outcomes | PPCM (n = 53) | Controls (n = 92) | p Value |
|---|---|---|---|
| Antepartum admission n, % | 18, 34.0 | 17, 18.5 | 0.001 |
| Preeclampsia n, % Pregnancy induced hypertension n, % | 10, 18.9 1, 1.9 | 11, 12.0 0, 0.0 | 0.08 0.37 |
| Gestational diabetes n, % | 8, 15.1 | 7, 7.7 | 0.12 |
| Chronic abruption n, % | 1, 1.9 | 1, 1.1 | 0.52 |
| Placenta previa n, % | 0, 0.0 | 2, 2.2 | 0.36 |
| Preterm labor, PPROM n, % | 12, 27.3 | 47, 51.1 | 0.001 |
| Intrauterine fetal demise n, % | 1, 1.9 | 1, 1.1 | 0.69 |
| Ectopic pregnancy n, % | 1, 1.9 | 0, 0.0 | 0.19 |
| Termination of pregnancy n, % | 1, 1.9 | 0, 0.0 | 0.19 |
| Intrapartum maternal outcomes | |||
| Cesarean section n, % | 34, 65.4 | 28, 30.4 | 0.001 |
| Post-partum hemorrhage n, % | 5, 9.4 | 2, 2.2 | 0.05 |
| Placental abruption n, % | 2, 3.8 | 2, 2.2 | 0.57 |
| Chorioamnionitis n, % | 1, 1.9 | 2, 2.2 | 0.91 |
| Placenta accreta spectrum n, % | 1, 1.9 | 1, 1.1 | 0.69 |
| Eclampsia n, % | 0, 0 | 1, 1.1 | 0.45 |
| Labor dystocia n, % | 0, 0 | 1, 1.1 | 0.45 |
| Non-cardiac maternal outcomes 12 months postpartum | |||
| Abscess, sepsis, infection n, % | 3, 5.7 | 1, 1.1 | 0.14 |
| Stroke, DVT, PE n, % | 2, 3.8 | 1, 1.1 | 0.55 |
| Bleeding, incisional n, % | 0, 0.0 | 1, 1.1 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfe, D.S.; Liu, C.; Alboucai, J.; Karten, A.; Mushi, J.; Yellin, S.; Berkowitz, J.L.; Vega, S.; Felix, N.; Liaqat, W.; et al. Maternal Outcomes in Women with Peripartum Cardiomyopathy versus Age and Race-Matched Peers in an Urban US Community. J. Cardiovasc. Dev. Dis. 2022, 9, 250. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd9080250
Wolfe DS, Liu C, Alboucai J, Karten A, Mushi J, Yellin S, Berkowitz JL, Vega S, Felix N, Liaqat W, et al. Maternal Outcomes in Women with Peripartum Cardiomyopathy versus Age and Race-Matched Peers in an Urban US Community. Journal of Cardiovascular Development and Disease. 2022; 9(8):250. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd9080250
Chicago/Turabian StyleWolfe, Diana S., Christina Liu, Jack Alboucai, Ariel Karten, Juliet Mushi, Shira Yellin, Julia L. Berkowitz, Shayna Vega, Nicole Felix, Wasla Liaqat, and et al. 2022. "Maternal Outcomes in Women with Peripartum Cardiomyopathy versus Age and Race-Matched Peers in an Urban US Community" Journal of Cardiovascular Development and Disease 9, no. 8: 250. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd9080250