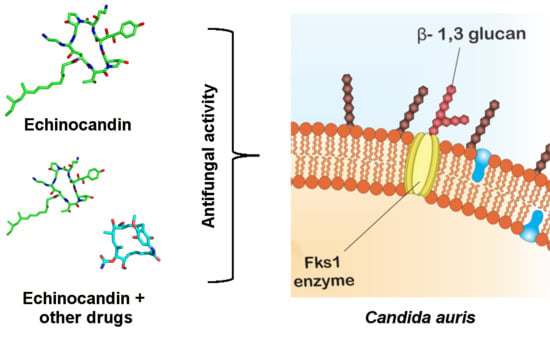
Echinocandins as Biotechnological Tools for Treating Candida auris Infections
Abstract
:1. Introduction
2. Echinocandins as Tools for Treating Candida auris Infections
3. Alternative Therapies and Clinical Applicability
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| AFLP | Amplified Fragment Length Polymorphism |
| FDA | The Food and Drug Administration |
| FKS | β-1,3-glucan synthase genes |
| ICU | Intensive Care Units |
| ITS | Internal Transcribed Spacer |
| MALDI-ToF | Matrix-Assisted Laser Desorption Ionization–Time of Flight |
| MFS | Major Facilitator Superfamily |
| MIC | Minimal Inhibition Concentration |
| PCR | Polymerase Chain Reaction |
| RHO1 | GTP-Binding protein precursor |
| STR | Short Tandem Repeat Typing |
References
- Chowdhary, A.; Anil Kumar, V.; Sharma, C.; Prakash, A.; Agarwal, K.; Babu, R.; Dinesh, K.R.; Karim, S.; Singh, S.K.; Hagen, F.; et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Wickes, B.L. Analysis of a Candida auris Outbreak Provides New Insights into an Emerging Pathogen. J. Clin. Microbiol. 2020, 58, e02083-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgacs, L.; Borman, A.M.; Prepost, E.; Toth, Z.; Kardos, G.; Kovacs, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Chow, N.A.; Gade, L.; Tsay, S.V.; Forsberg, K.; Greenko, J.A.; Southwick, K.L.; Barrett, P.M.; Kerins, J.L.; Lockhart, S.R.; Chiller, T.M.; et al. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: A molecular epidemiological survey. Lancet Infect. Dis. 2018, 18, 1377–1384. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Kim, M.N.; Shin, J.H.; Sung, H.; Lee, K.; Kim, E.C.; Ryoo, N.; Lee, J.S.; Jung, S.I.; Park, K.H.; Kee, S.J.; et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: Identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 2009, 48, e57–e61. [Google Scholar] [CrossRef] [Green Version]
- Emara, M.; Ahmad, S.; Khan, Z.; Joseph, L.; Al-Obaid, I.; Purohit, P.; Bafna, R. Candida auris candidemia in Kuwait, 2014. Emerg. Infect. Dis. 2015, 21, 1091–1092. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Khan, Z.; Al-Sweih, N.; Alfouzan, W.; Joseph, L. Candida auris in various hospitals across Kuwait and their susceptibility and molecular basis of resistance to antifungal drugs. Mycoses 2020, 63, 104–112. [Google Scholar] [CrossRef]
- Magobo, R.E.; Corcoran, C.; Seetharam, S.; Govender, N.P. Candida auris-associated candidemia, South Africa. Emerg. Infect. Dis. 2014, 20, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Melo, A.S.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitan, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-Lopez, A.I.; Martinez-Morel, H.; Calabuig, E.; Salavert-Lleti, M.; Ramirez, P.; Lopez-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsin, J.; Hagen, F.; Al-Balushi, Z.A.M.; de Hoog, G.S.; Chowdhary, A.; Meis, J.F.; Al-Hatmi, A.M.S. The first cases of Candida auris candidaemia in Oman. Mycoses 2017, 60, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, R.; Berman, J.; Novikov, A.; Bash, E.; Shachor-Meyouhas, Y.; Zakin, S.; Maor, Y.; Tarabia, J.; Schechner, V.; Adler, A.; et al. Multidrug-Resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg. Infect. Dis. 2017, 23, 195–203. [Google Scholar] [CrossRef]
- Morales-Lopez, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzon, A.; Martinez, H.P.; Rodriguez, G.J.; Alvarez-Moreno, C.A.; Rodriguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef] [Green Version]
- Arauz, A.B.; Caceres, D.H.; Santiago, E.; Armstrong, P.; Arosemena, S.; Ramos, C.; Espinosa-Bode, A.; Borace, J.; Hayer, L.; Cedeno, I.; et al. Isolation of Candida auris from 9 patients in Central America: Importance of accurate diagnosis and susceptibility testing. Mycoses 2018, 61, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med. Mycol. 2017, 55, 563–567. [Google Scholar] [CrossRef]
- Lane, C.R.; Seemann, T.; Worth, L.J.; Easton, M.; Pitchers, W.; Wong, J.; Cameron, D.; Azzato, F.; Bartolo, R.; Mateevici, C.; et al. Incursions of Candida auris into Australia, 2018. Emerg. Infect. Dis. 2020, 26, 1326–1328. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Almaghrabi, R.; Althawadi, S.; Omrani, A. First report of Candida auris infections from Saudi Arabia. J. Infect. Public Health 2018, 11, 598–599. [Google Scholar] [CrossRef]
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles in Vitro. J. Clin. Microbiol. 2019, 57, e02055-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Derkacz, D.; Muraszko, J.; Panek, J.J.; Jezierska, A.; Lukaszewicz, M.; Krasowska, A. Fluconazole and Lipopeptide Surfactin Interplay during Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure. Pharmaceutics 2020, 12, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chybowska, A.D.; Childers, D.S.; Farrer, R.A. Nine Things Genomics Can Tell Us about Candida auris. Front. Genet. 2020, 11, 351. [Google Scholar] [CrossRef]
- Biniarz, P.; Baranowska, G.; Feder-Kubis, J.; Krasowska, A. The lipopeptides pseudofactin II and surfactin effectively decrease Candida albicans adhesion and hydrophobicity. Antonie Van Leeuwenhoek 2015, 108, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W. Lipopeptides: From self-assembly to bioactivity. Chem. Commun. 2015, 51, 8574–8583. [Google Scholar] [CrossRef] [Green Version]
- Mroczynska, M.; Brillowska-Dabrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Perlin, D.S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Update 2007, 10, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.M.; Jain, R.; Danziger, L.H. Micafungin: A new echinocandin antifungal. Pharmacotherapy 2007, 27, 53–67. [Google Scholar] [CrossRef]
- Vardanyan, R.; Hruby, V. Antifungal Drugs. In Synthesis of Best-Seller Drugs; Press, A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, p. 868. [Google Scholar]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Qadota, H.; Python, C.P.; Inoue, S.B.; Arisawa, M.; Anraku, Y.; Zheng, Y.; Watanabe, T.; Levin, D.E.; Ohya, Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 1996, 272, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.F. What is known about Candida auris. JAMA 2019. [Google Scholar] [CrossRef] [PubMed]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, H.; Nishimura, Y. Cyclic Lipopeptide Antibiotics. Stud. Nat. Prod. Chem. 2008, 35, 693–751. [Google Scholar]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S604–S611. [Google Scholar] [CrossRef] [Green Version]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The newest class of antifungals. Ann. Pharmacother. 2009, 43, 1647–1657. [Google Scholar] [CrossRef]
- Dudiuk, C.; Berrio, I.; Leonardelli, F.; Morales-Lopez, S.; Theill, L.; Macedo, D.; Yesid-Rodriguez, J.; Salcedo, S.; Marin, A.; Gamarra, S.; et al. Antifungal activity and killing kinetics of anidulafungin, caspofungin and amphotericin B against Candida auris. J. Antimicrob. Chemother. 2019, 74, 2295–2302. [Google Scholar] [CrossRef]
- Romera, D.; Aguilera-Correa, J.J.; Gadea, I.; Vinuela-Sandoval, L.; Garcia-Rodriguez, J.; Esteban, J. Candida auris: A comparison between planktonic and biofilm susceptibility to antifungal drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Balkovec, J.M.; Hughes, D.L.; Masurekar, P.S.; Sable, C.A.; Schwartz, R.E.; Singh, S.B. Discovery and development of first in class antifungal caspofungin (CANCIDAS(R))—A case study. Nat. Prod. Rep. 2014, 31, 15–34. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [PubMed] [Green Version]
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hager, C.L.; Larkin, E.L.; Long, L.A.; Ghannoum, M.A. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J. Antimicrob. Chemother. 2018, 73, 2085–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, B.R.; James, K.D.; Polowy, K.; Bryant, B.J.; Vaidya, A.; Smith, S.; Laudeman, C.P. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J. Antibiot. 2017, 70, 130–135. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Activity of CD101, a long-acting echinocandin, against clinical isolates of Candida auris. Diagn Microbiol. Infect. Dis. 2018, 90, 196–197. [Google Scholar] [CrossRef]
- Lepak, A.J.; Zhao, M.; Andes, D.R. Pharmacodynamic Evaluation of Rezafungin (CD101) against Candida auris in the Neutropenic Mouse Invasive Candidiasis Model. Antimicrob. Agents Chemother. 2018, 62, e01572-18. [Google Scholar] [CrossRef] [Green Version]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S612–S617. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, H.C.; Monteiro, M.C.; Rossi, S.A.; Peman, J.; Ruiz-Gaitan, A.; Mendes-Giannini, M.J.S.; Mellado, E.; Zaragoza, O. Identification of Off-Patent Compounds That Present Antifungal Activity against the Emerging Fungal Pathogen Candida auris. Front. Cell Infect. Microbiol. 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017, 61, e01056-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, S.; Rezaie, S.; Daie Ghazvini, R.; Hashemi, S.J.; Badali, H.; Foroumadi, A.; Diba, K.; Chowdhary, A.; Meis, J.F.; Khodavaisy, S. In Vitro Interaction of Geldanamycin with Triazoles and Echinocandins against Common and Emerging Candida Species. Mycopathologia 2019, 184, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.C.; Ferreira, L.; Petersen, A.; Dias, B.R.S.; Menezes, J.P.B.; Moreira, D.R.M.; Hernandes, M.Z.; Veras, P.S.T. A docking-based structural analysis of geldanamycin-derived inhibitor binding to human or Leishmania Hsp90. Sci. Rep. 2019, 9, 14756. [Google Scholar] [CrossRef] [PubMed]
- Nagy, F.; Toth, Z.; Daroczi, L.; Szekely, A.; Borman, A.M.; Majoros, L.; Kovacs, R. Farnesol increases the activity of echinocandins against Candida auris biofilms. Med. Mycol. 2020, 58, 404–407. [Google Scholar] [CrossRef]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; Lopez-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [Green Version]
- Katragkou, A.; McCarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-Rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 2392–2401. [Google Scholar] [CrossRef] [Green Version]
- Scheper, M.A.; Shirtliff, M.E.; Meiller, T.F.; Peters, B.M.; Jabra-Rizk, M.A. Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia 2008, 10, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, R.; Bozo, A.; Gesztelyi, R.; Doman, M.; Kardos, G.; Nagy, F.; Toth, Z.; Majoros, L. Effect of caspofungin and micafungin in combination with farnesol against Candida parapsilosis biofilms. Int. J. Antimicrob. Agents 2016, 47, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Zeidler, U.; Bougnoux, M.E.; Lupan, A.; Helynck, O.; Doyen, A.; Garcia, Z.; Sertour, N.; Clavaud, C.; Munier-Lehmann, H.; Saveanu, C.; et al. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J. Antimicrob. Chemother. 2013, 68, 1285–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 ), whereas multiple cases are represented as (
), whereas multiple cases are represented as (  ). (
). (  ) Represents outbreak reports. The timeline presents the first report in each country highlighted over the years, with 2009 being the year in which the first case was reported.
) Represents outbreak reports. The timeline presents the first report in each country highlighted over the years, with 2009 being the year in which the first case was reported.
 ), whereas multiple cases are represented as (
), whereas multiple cases are represented as (  ). (
). (  ) Represents outbreak reports. The timeline presents the first report in each country highlighted over the years, with 2009 being the year in which the first case was reported.
) Represents outbreak reports. The timeline presents the first report in each country highlighted over the years, with 2009 being the year in which the first case was reported.

| Name | PubChem CID | Molecular Formula | Molecular Weight (g/mol) |
|---|---|---|---|
| 1-((4R,5R)-4,5-dihydroy-L-ornithine) Echinocandin B | 134693052 | C34H51N7O15 | 797.8 |
| 1029890-89-8 | 134693051 | C34H52CIN7O15 | 843.3 |
| 143131-16-2 Echinocandin B | 456858 | C53H88N8O16 | 1093.3 |
| 166663-25-8 | 15224271 | C58H73N7O17 | 1140.2 |
| 79404-91-4 | 71762 | C49H71N7O17 | 1030.099 |
| 79411-15-7 | 91632900 | C34H51N7O15 | 797.8 |
| Aminocandin | 160772305 | C56H79N9O13 | 1086.3 |
| Anidulafungin | 166548 | C58H73N7O17 | 1140.2 |
| Biafungin | 92135635 | C63H85N8O17 + | 1226.4 |
| Caspofungin | 2826718 | C52H88N10O15 | 1093.3 |
| CHEBI:2450 | 53297328 | C51H82N8O17 | 1079.2 |
| Cilofungin and Amphotericin B (AmB) | 6473895 | C96H144N8O34 | 1954.2 |
| CINH3EtCOO Echinocandin | 11984605 | C53H86CIN9O18 | 1172.8 |
| CINH3MeCOO Echinocandin | 11984607 | C52H84CIN9O18 | 1158.7 |
| DiMeNEtOCOO Echinocandin | 456505 | C55H89N9O19 | 1180.3 |
| Echinocandin B | 9898144 | C52H81N7O16 | 1060.2 |
| Echinocandin B Nucleus | 91820167 | C34H52N7O15 + | 798.8 |
| Echinocandin B Nucleus Hydrochloride | 138115264 | C34H52CIN7O15 | 834.3 |
| Echinocandin C | 10260509 | C52H81N7O15 | 1044.2 |
| Echinocandin D | 12773979 | C52H81N7O13 | 1012.2 |
| Echinocandin Phosphate | 23715870 | C50H80N8NaO20P | 1167.2 |
| HOOCEtNHCOO Echinocandin | 456500 | C54H85N9O20 | 1180.3 |
| HOOCMeNHCOO Echinocandin | 456501 | C53H83N9O20 | 1166.3 |
| HOOCMeNMeCOO Echinocandin | 456502 | C54H85N9O20 | 1180.3 |
| HOOCPrCOO Echinocandin | 456504 | C55H86N8O20 | 1179.3 |
| L 731373 | 462493 | C50H82N8O16 | 1051.2 |
| Lipopeptide Der A-2a | 456855 | C50H79N8Na2O18 | 1157.2 |
| Micafungin | 477468 | C56H71N9O23S | 1270.3 |
| Mulundocandin | 121225706 | C48H77N7O16 | 1008.2 |
| Pneumocandin B0 | 5742645 | C50H80N8O17 | 1065.2 |
| Rezafungin | 78318119 | C63H85N8O17 + | 1226.4 |
| Tetrahydroechinocandin B | 171361 | C52H85N7O16 | 1064.3 |
| YKPHLXGEPNYRPY-UHFFFAOYSA-N | 122233 | C50H81N7O16 | 1036.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cândido, E.d.S.; Affonseca, F.; Cardoso, M.H.; Franco, O.L. Echinocandins as Biotechnological Tools for Treating Candida auris Infections. J. Fungi 2020, 6, 185. https://0-doi-org.brum.beds.ac.uk/10.3390/jof6030185
Cândido EdS, Affonseca F, Cardoso MH, Franco OL. Echinocandins as Biotechnological Tools for Treating Candida auris Infections. Journal of Fungi. 2020; 6(3):185. https://0-doi-org.brum.beds.ac.uk/10.3390/jof6030185
Chicago/Turabian StyleCândido, Elizabete de Souza, Flávia Affonseca, Marlon Henrique Cardoso, and Octavio Luiz Franco. 2020. "Echinocandins as Biotechnological Tools for Treating Candida auris Infections" Journal of Fungi 6, no. 3: 185. https://0-doi-org.brum.beds.ac.uk/10.3390/jof6030185