Immunological Aspects of Chytridiomycosis
Abstract
:1. Introduction
2. Constitutive Defences Are Sufficient to Prevent Infection in Many Cases
2.1. Skin Microbiota
2.2. Antimicrobial Peptides, Defensive Enzymes and Natural Mucosal Antibodies
2.3. Skin Sloughing
3. The Induced Innate Immune Response Appears Inadequate to Resolve Batrachochytrium dendrobatidis Infection
3.1. Classical Pathogen Recognition Pathways May Be Impaired for Infections by Batrachochytrium dendrobatidis
3.2. Intracellular Immunoevasion and Insufficiently Effective Cell-Autonomous Immunity
3.3. Proinflammatory Responses Are Mild or Absent, Possibly Due to Batrachochytrium dendrobatidis Immunosuppression
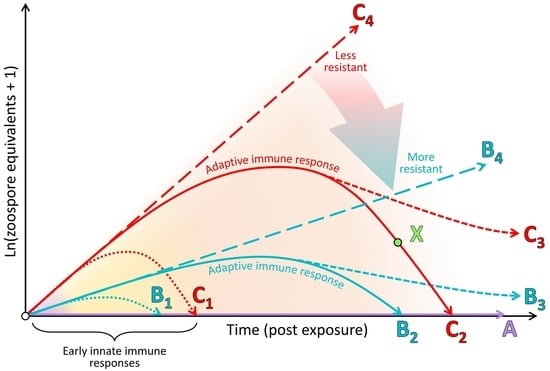
4. The Adaptive Immune Response Is Compromised by Batrachochytrium dendrobatidis-Associated Immunosuppression
4.1. The Amphibian Adaptive Immune System Can Respond to Batrachochytrium dendrobatidis
4.2. The Clinical Adaptive Immune Response Is Generally Poor and Nonprotective
4.3. Metabolites Produced by Batrachochytrium dendrobatidis Suppress Lymphocytes (Particularly T Cells)
4.4. Immunopathology Due to Ineffective Immune Responses Likely Plays a Key Role in Morbidity
5. Conclusions and Areas for Further Research
- Prevention is the best cure, and mitigation strategies aimed at enhancing constitutive defences should remain a high priority for populations declining in situ [97].
- Comparative kinetic analyses of the early innate immune response (e.g., especially 8–72 h post exposure) using single-cell transcriptomics of localised infected skin versus uninfected skin may help to characterise the dynamic expression of immune-related genes and factors through time.
- The role and efficacy of cell-autonomous immunity should be explored further, particularly with regard to interferon-induced GTPases, the inflammasome and whether cell death pathways are associated with host defence or Bd virulence.
- The role and potential efficacy of immune memory (both innate and adaptive) should be examined, particularly with regard to amphibian populations in the wild. Habitats that experience temperature fluctuations may permit repeated nonlethal exposures to Bd, potentially enhancing immune memory. Work should also continue to explore whether long-term immunity can be induced in long-lived species released into the wild.
- Most work to date has focused on adult amphibians, although metamorphs have frequently been identified as being a more susceptible life stage [128,183]. Improved understanding of the factors involved in determining metamorph survival and the role of different immune components in survival would be beneficial.
- Our understanding of the immune response to Bsal is still preliminary and would benefit from identifying the similarities and differences between Bd and Bsal immune responses (reviewed by [23]).
- Immunogenetic studies on the MHC locus have revealed promising results concerning the capacity for amphibians to evolve resistance to infection [9]. Nevertheless, MHC provides only part of the story with respect to heritable changes, and genome-wide association studies may provide a more complete picture [157].
Author Contributions
Funding
Conflicts of Interest
References
- Monastersky, R. Biodiversity: Life-a status report. Nature 2014, 516, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [Green Version]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrochochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef] [Green Version]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 2007, 4, 125–134. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hunter, D.A.; Grogan, L.; Berger, L.; Kolby, J.; McFadden, M.; Marantelli, G.; Skerratt, L.F.; Driscoll, D.A. Interventions for reducing extinction risk in chytridiomycosis-threatened amphibians. Conserv. Biol. 2014, 28, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Savage, A.E.; Zamudio, K.R. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc. R. Soc. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [Green Version]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Rotroff, S.I.; Ntinou, M. Industrial Religion: The Saucer Pyres of the Athenian Agora. Hesperia Suppl. 2013, 47, iii-228. [Google Scholar]
- Money, N.P. Fungal Diversity. In The fungi, 3rd ed.; Boddy, L., Watkinson, S.C., Money, N.P., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 1–36. [Google Scholar]
- Olson, D.H.; Aanensen, D.M.; Ronnenberg, K.L.; Powell, C.I.; Walker, S.F.; Bielby, J.; Garner, T.W.J.; Weaver, G.; Fisher, M.C. Mapping the Global Emergence of Batrachochytrium dendrobatidis, the Amphibian Chytrid Fungus. PLoS ONE 2013, 8, e56802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.C.; Garner, T.W.J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 2020, 18, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Farrer, R.A. Batrachochytrium salamandrivorans. Trends Microbiol. 2019, 27, 892–893. [Google Scholar] [CrossRef]
- Waddle, J.H.; Grear, D.A.; Mosher, B.A.; Grant, E.H.C.; Adams, M.J.; Backlin, A.R.; Barichivich, W.J.; Brand, A.B.; Bucciarelli, G.M.; Calhoun, D.L.; et al. Batrachochytrium salamandrivorans (Bsal) not detected in an intensive survey of wild North American amphibians. Sci. Rep. 2020, 10, 13012. [Google Scholar] [CrossRef]
- Richgels, K.L.D.; Russell, R.E.; Adams, M.J.; White, C.L.; Grant, E.H.C. Spatial variation in risk and consequence of Batrachochytrium salamandrivorans introduction in the USA. R. Soc. Open Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, A.V.; Fleischer, R.C.; Lips, K.R. Double trouble: Co-infections of chytrid fungi will severely impact widely distributed newts. Biol. Invasions 2019. [Google Scholar] [CrossRef]
- McDonald, C.A.; Longo, A.V.; Lips, K.R.; Zamudio, K.R. Incapacitating effects of fungal coinfection in a novel pathogen system. Mol. Ecol. 2020. [Google Scholar] [CrossRef]
- Farrer, R.A.; Martel, A.; Verbrugghe, E.; Abouelleil, A.; Ducatelle, R.; Longcore, J.E.; James, T.Y.; Pasmans, F.; Fisher, M.C.; Cuomo, C.A. Genomic innovations linked to infection strategies across emerging pathogenic chytrid fungi. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Barnhart, K.; Bletz, M.C.; LaBumbard, B.; Tokash-Peters, A.; Gabor, C.R.; Woodhams, D.C. Batrachochytrium salamandrivorans elicits acute stress response in spotted salamanders but not infection or mortality. Anim. Conserv. 2020, 23, 533–546. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. Global amphibian declines, disease, and the ongoing battle between Batrachochytrium fungi and the immune system. Herpetologica 2020, 76, 178–188. [Google Scholar] [CrossRef]
- Berger, L.; Hyatt, A.D.; Speare, R.; Longcore, J.E. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2005, 68, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marantelli, G.; Berger, L.; Speare, R.; Keegan, L. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac. Conserv. Biol. 2004, 10, 173–179. [Google Scholar] [CrossRef]
- Berger, L.; Longcore, J.E.; Speare, R.; Hyatt, A.; Skerratt, L.F. Chapter 2: Fungal Diseases in Amphibians. In Amphibian Biology, Volume 8: Amphibian Decline: Disease, Parasites, Maladies, and Pollution; Heatwole, H., Wilkinson, J.W., Eds.; Surrey Beatty & Sons: Chipping Norton, NSW, Australia, 2009; Volume 8, pp. 2988–3052. [Google Scholar]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F.; et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmer, M.E.B.; Cramp, R.L.; Russo, C.J.M.; White, C.R.; Franklin, C.E. Skin sloughing in susceptible and resistant amphibians regulates infection with a fungal pathogen. Sci. Rep. 2017, 7, 3529. [Google Scholar] [CrossRef] [Green Version]
- Grogan, L.F.; Skerratt, L.F.; Berger, L.; Cashins, S.D.; Trengove, R.D.; Gummer, J.P.A. Chytridiomycosis causes catastrophic organism-wide metabolic dysregulation including profound failure of cellular energy pathways. Sci. Rep. 2018, 8, 8188. [Google Scholar] [CrossRef]
- Rosenblum, E.B.; James, T.Y.; Zamudio, K.R.; Poorten, T.J.; Ilut, D.; Rodriguez, D.; Eastman, J.M.; Richards-Hrdlicka, K.; Joneson, S.; Jenkinson, T.S.; et al. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc. Natl. Acad. Sci. USA 2013, 110, 9385–9390. [Google Scholar] [CrossRef] [Green Version]
- Farrer, R.A.; Weinert, L.A.; Bielby, J.; Garner, T.W.J.; Balloux, F.; Clare, F.; Bosch, J.; Cunningham, A.A.; Weldon, C.; Du Preez, L.H.; et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl. Acad. Sci. USA 2011, 108, 18732–18736. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Badhon, M.K.; Salauddin, M.; Rabbe, M.F.; Islam, M.S. Chytrid infection in Asia: How much do we know and what else do we need to know? Herpetol. J. 2020, 30, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.; Liu, X.; Fisher, M.C.; Garner, T.W.J.; Li, Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers. Distrib. 2012, 18, 307–318. [Google Scholar] [CrossRef]
- Voyles, J.; Woodhams, D.C.; Saenz, V.; Byrne, A.Q.; Perez, R.; Rios-Sotelo, G.; Ryan, M.J.; Bletz, M.C.; Sobell, F.A.; McLetchie, S.; et al. Shifts in disease dynamics in a tropical amphibian assemblage are not due to pathogen attenuation. Science 2018, 359, 1517–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haydon, D.T.; Cleaveland, S.; Taylor, L.H.; Laurenson, M.K. Identifying reservoirs of infection: A conceptual and practical challenge. Emerg. Infect. Dis. 2002, 8, 1468–1473. [Google Scholar]
- Daszak, P.; Strieby, A.; Cunningham, A.A.; Longcore, J.E.; Brown, C.C.; Porter, D. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J. 2004, 14, 201–207. [Google Scholar]
- Brannelly, L.A.; Webb, R.J.; Hunter, D.A.; Clemann, N.; Howard, K.; Skerratt, L.F.; Berger, L.; Scheele, B.C. Non-declining amphibians can be important reservoir hosts for amphibian chytrid fungus. Anim. Conserv. 2017, 21, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Scheele, B.C.; Hunter, D.A.; Brannelly, L.A.; Skerratt, L.F.; Driscoll, D.A. Reservoir-host amplification of disease impact in an endangered amphibian. Conserv. Biol. 2017, 31, 592–600. [Google Scholar] [CrossRef]
- Newell, D.A.; Goldingay, R.L.; Brooks, L.O. Population Recovery following Decline in an Endangered Stream-Breeding Frog (Mixophyes fleayi) from Subtropical Australia. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, R.; Alvarado, G. Craugastor escoces (Anura: Craugastoridae) reappears after 30 years: Rediscovery of an “extinct” Neotropical frog. Amphibia-Reptilia 2017, 38. [Google Scholar] [CrossRef]
- Edholm, E.-S.; Banach, M.; Hyoe Rhoo, K.; Pavelka, M.S.; Robert, J. Distinct MHC class I-like interacting invariant T cell lineage at the forefront of mycobacterial immunity uncovered in Xenopus. Proc. Natl. Acad. Sci. USA 2018, 115, E4023–E4031. [Google Scholar] [CrossRef] [Green Version]
- Robert, J. Experimental platform using the amphibian xenopus laevis for research in fundamental and medical immunology. Cold Spring Harb. Protoc. 2020, 2020, 247–251. [Google Scholar] [CrossRef]
- Du Pasquier, L.; Schwager, J.; Flajnik, M.F. The immune system of Xenopus. Annu. Rev. Immunol. 1989, 7, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Ohta, Y. Comparative and Developmental Study of the Immune System in Xenopus. Dev. Dyn. 2009, 238, 1249–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, I.S.; Roubos, E.W.; Mangoni, M.L.; Yoshizato, K.; Vaudry, H.; Kloepper, J.E.; Pattwell, D.M.; Maderson, P.F.A.; Paus, R. From frog integument to human skin: Dermatological perspectives from frog skin biology. Biol. Rev. 2014, 89, 618–655. [Google Scholar] [CrossRef]
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453. [Google Scholar] [CrossRef]
- Marr, S.; Morales, H.; Bottaro, A.; Cooper, M.; Flajnik, M.; Robert, J. Localization and differential expression of Activation-Induced Cytidine Deaminase in the amphibian Xenopus upon antigen stimulation and during early development. J. Immunol. 2007, 179, 6783–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, E.; Bininda-Emonds, O.R.P.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Ladram, A.; Nicolas, P. Antimicrobial peptides from frog skin: Biodiversity and therapeutic promises. Front. Biosci. Landmark 2016, 21, 1341–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol. Rev. 1998, 162, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Maniero, G.D.; Robert, J. Phylogenetic conservation of gp96-mediated antigen-specific cellular immunity: New evidence from adoptive cell transfer in Xenopus. Transplantation 2004, 78, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barreda, D.R.; Zhang, Y.A.; Boshra, H.; Gelman, A.E.; LaPatra, S.; Tort, L.; Sunyer, J.O. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 2006, 7, 1116–1124. [Google Scholar] [CrossRef]
- Mashoof, S.; Goodroe, A.; Du, C.C.; Eubanks, J.O.; Jacobs, N.; Steiner, J.M.; Tizard, I.; Suchodolski, J.S.; Criscitiello, M.F. Ancient T-independence of mucosal IgX/A: Gut microbiota unaffected by larval thymectomy in Xenopus laevis. Mucosal Immunol. 2013, 6, 358–368. [Google Scholar] [CrossRef]
- Mußmann, R.; Du Pasquier, L.; Hsu, E. Is Xenopus IgX an analog of IgA? Eur. J. Immunol. 1996, 26, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Warr, G.W.; Magor, K.E.; Higgins, D.A. IgY: Clues to the origins of modern antibodies. Immunol. Today 1995, 16, 392–398. [Google Scholar] [CrossRef]
- Colombo, B.M.; Scalvenzi, T.; Benlamara, S.; Pollet, N. Microbiota and mucosal immunity in amphibians. Front. Immunol. 2015, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Brattstrom, B.H. Amphibian temperature regulation studies in the field and laboratory. Integr. Comp. Biol. 1979, 19, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Dix, N.J.; Webster, J. Fungi of Extreme Environments. In Fungal Ecol.; Dix, N.J., Webster, J., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 322–340. [Google Scholar] [CrossRef]
- Robert, V.A.; Casadevall, A. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 2009, 200, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; The Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Longcore, J.E.; Simmons, D.R. Chytridiomycota. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Humphrey, J. Mucormycosis in a free-ranging green tree frog from Australia. J. Wildl. Dis. 1997, 33, 903–907. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.; Skerratt, L.F.; Zhu, X.Q.; Young, S.; Speare, R. Severe sparganosis in australian tree frogs. J. Wildl. Dis. 2009, 45, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef]
- Stevenson, L.A.; Alford, R.A.; Bell, S.C.; Roznik, E.A.; Berger, L.; Pike, D.A. Variation in Thermal Performance of a Widespread Pathogen, the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, K.A.; Retallick, R.W.R.; Puschendorf, R.; Skerratt, L.F.; Rosauer, D.; McCallum, H.I.; Berger, L.; Speare, R.; VanDerWal, J. Assessing spatial patterns of disease risk to biodiversity: Implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. J. Appl. Ecol. 2011, 48, 163–173. [Google Scholar] [CrossRef]
- Hettyey, A.; Ujszegi, J.; Herczeg, D.; Holly, D.; Voros, J.; Schmidt, B.R.; Bosch, J. Mitigating Disease Impacts in Amphibian Populations: Capitalizing on the Thermal Optimum Mismatch Between a Pathogen and Its Host. Front. Ecol. Evol. 2019, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Roznik, E.A.; Alford, R.A. Seasonal ecology and behavior of an endangered rainforest frog (Litoria rheocola) threatened by disease. PLoS ONE 2015, 10, e0127851. [Google Scholar] [CrossRef]
- Lips, K.R. Decline of a tropical montane amphibian fauna. Conserv. Biol. 1998, 12, 106–117. [Google Scholar] [CrossRef]
- La Marca, E.; Lips, K.R.; Lötters, S.; Puschendorf, R.; Ibáñez, R.; Rueda-Almonacid, J.V.; Schulte, R.; Marty, C.; Castro, F.; Manzanilla-Puppo, J.; et al. Catastrophic Population Declines and Extinctions in Neotropical Harlequin Frogs (Bufonidae: Atelopus). Biotropica 2005, 37, 190–201. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Woodhams, D.C. Amphibian immunity: Staying in tune with the environment. In Ecoimmunology; Demas, G., Nelson, R., Eds.; Oxford University press: Oxford, UK, 2011; pp. 92–143. [Google Scholar]
- Robak, M.J.; Reinert, L.K.; Rollins-Smith, L.A.; Richards-Zawacki, C.L. Out in the cold and sick: Low temperatures and fungal infections impair a frog’s skin defenses. J. Exp. Biol. 2019, 222, jeb209445. [Google Scholar] [CrossRef] [Green Version]
- Terrell, K.A.; Quintero, R.P.; Murray, S.; Kleopfer, J.D.; Murphy, J.B.; Evans, M.J.; Nissen, B.D.; Gratwicke, B. Cryptic impacts of temperature variability on amphibian immune function. J. Exp. Biol. 2013, 216, 4204–4211. [Google Scholar] [CrossRef] [Green Version]
- Moretti, E.H.; Titon, S.C.M.; Titon, B.; Marques, F.S.; Gomes, F.R. Thermal sensitivity of innate immune response in three species of Rhinella toads. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2019, 237. [Google Scholar] [CrossRef]
- Ellison, A.; Zamudio, K.; Lips, K.; Muletz-Wolz, C. Temperature-mediated shifts in salamander transcriptomic responses to the amphibian-killing fungus. Mol. Ecol. 2020, 29, 325–343. [Google Scholar] [CrossRef]
- Raffel, T.R.; Rohr, J.R.; Kiesecker, J.M.; Hudson, P.J. Negative effects of changing temperature on amphibian immunity under field conditions. Funct. Ecol. 2006, 20, 819–828. [Google Scholar] [CrossRef]
- Andre, S.E.; Parker, J.; Briggs, C.J. Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the mountain yellow-legged frog (Rana muscosa). J. Wildl. Dis. 2008, 44, 716–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonn, J.M.; Berman, S.; Richards-Zawacki, C.L. The Influence of Temperature on Chytridiomycosis In Vivo. EcoHealth 2017, 14, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J.; St.-Hilaire, S.; Corn, P.S. Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis. Aquat. Org. 2011, 95, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, J.R.; Raffel, T.R.; Romansic, J.M.; McCallum, H.; Hudson, P.J. Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl. Acad. Sci. USA 2008, 105, 17436–17441. [Google Scholar] [CrossRef] [Green Version]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef] [Green Version]
- Grogan, L.F.; Robert, J.; Berger, L.; Skerratt, L.F.; Scheele, B.C.; Castley, J.G.; Newell, D.A.; McCallum, H.I. Review of the amphibian immune response to chytridiomycosis, and future directions. Front. Immunol. 2018, 9, 2536. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Garland Science, Taylor & Francis Group, LLC: New York, NY, USA, 2017. [Google Scholar]
- Varga, J.F.A.; Bui-Marinos, M.P.; Katzenback, B.A. Frog Skin Innate Immune Defences: Sensing and Surviving Pathogens. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef]
- Bagnara, J.T.; Taylor, J.D.; Hadley, M.E. The dermal chromatophore unit. J. Cell Biol. 1968, 38, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.; Grosso, A.; De Sousa, R.C. The amphibian epidermis: Distribution of mitochondria-rich cells and the effect of oxytocin. J. Cell Sci. 1981, 52, 197–213. [Google Scholar] [PubMed]
- Clarke, B.T. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. 1997, 72, 365–379. [Google Scholar] [CrossRef]
- Ribas, L.; Li, M.S.; Doddington, B.J.; Robert, J.; Seidel, J.A.; Simon Kroll, J.; Zimmerman, L.B.; Grassly, N.C.; Garner, T.W.J.; Fisher, M.C. Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parakkal, P.F.; Matoltsy, A.G. A study of the fine structure of the epidermis of Rana pipiens. J. Cell Biol. 1964, 20, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollins-Smith, L.A.; Ramsey, J.P.; Reinert, L.K.; Woodhams, D.C.; Livo, L.J.; Carey, C. Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front. Biosci. 2009, 1, 68–91. [Google Scholar] [CrossRef]
- Meyer, E.A.; Cramp, R.L.; Bernal, M.H.; Franklin, C.E. Changes in cutaneous microbial abundance with sloughing: Possible implications for infection and disease in amphibians. Dis. Aquat. Org. 2012, 101, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, J.P.; Reinert, L.K.; Harper, L.K.; Woodhams, D.C.; Rollins-Smith, L.A. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef] [Green Version]
- Pask, J.D.; Cary, T.L.; Rollins-Smith, L.A. Skin peptides protect juvenile leopard frogs (Rana pipiens) against chytridiomycosis. J. Exp. Biol. 2013, 216, 2908–2916. [Google Scholar] [CrossRef] [Green Version]
- Holden, W.M.; Hanlon, S.M.; Woodhams, D.C.; Chappell, T.M.; Wells, H.L.; Glisson, S.M.; McKenzie, V.J.; Knight, R.; Parris, M.J.; Rollins-Smith, L.A. Skin bacteria provide early protection for newly metamorphosed southern leopard frogs (Rana sphenocephala) against the frog-killing fungus, Batrachochytrium dendrobatidis. Biol. Conserv. 2015, 187, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Kueneman, J.G.; Parfrey, L.W.; Woodhams, D.C.; Archer, H.M.; Knight, R.; McKenzie, V.J. The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 2014, 23, 1238–1250. [Google Scholar] [CrossRef]
- West, A.G.; Waite, D.W.; Deines, P.; Bourne, D.G.; Digby, A.; McKenzie, V.J.; Taylor, M.W. The microbiome in threatened species conservation. Biol. Conserv. 2019, 229, 85–98. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Martínez-Ugalde, E.; Orta, A.H. The amphibian skin microbiome and its protective role against chytridiomycosis. Herpetologica 2020, 76, 167–177. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Lam, B.A.; Walton, D.B.; Harris, R.N. Motile zoospores of Batrachochytrium dendrobatidis move away from antifungal metabolites produced by amphibian skin bacteria. EcoHealth 2011, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kearns, P.J.; Fischer, S.; Fernández-Beaskoetxea, S.; Gabor, C.R.; Bosch, J.; Bowen, J.L.; Tlusty, M.F.; Woodhams, D.C. Fight fungi with fungi: Antifungal properties of the amphibian mycobiome. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.C.; LaBumbard, B.C.; Barnhart, K.L.; Becker, M.H.; Bletz, M.C.; Escobar, L.A.; Flechas, S.V.; Forman, M.E.; Iannetta, A.A.; Joyce, M.D.; et al. Prodigiosin, Violacein, and Volatile Organic Compounds Produced by Widespread Cutaneous Bacteria of Amphibians Can Inhibit Two Batrachochytrium Fungal Pathogens. Microb. Ecol. 2018, 75, 1049–1062. [Google Scholar] [CrossRef]
- Myers, J.M.; Ramsey, J.P.; Blackman, A.L.; Nichols, A.E.; Minbiole, K.P.C.; Harris, R.N. Synergistic inhibition of the lethal fungal pathogen Batrachochytrium dendrobatidis: The combined effect of symbiotic bacterial metabolites and antimicrobial peptides of the frog rana muscosa. J. Chem. Ecol. 2012, 38, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Loudon, A.H.; Holland, J.A.; Umile, T.P.; Burzynski, E.A.; Minbiole, K.P.C.; Harris, R.N. Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Bletz, M.C.; Kelly, M.; Sabino-Pinto, J.; Bales, E.; Van Praet, S.; Bert, W.; Boyen, F.; Vences, M.; Steinfartz, S.; Pasmans, F.; et al. Disruption of skin microbiota contributes to salamander disease. Proc. R. Soc. B Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Kueneman, J.G.; Woodhams, D.C.; Harris, R.; Archer, H.M.; Knight, R.; McKenzie, V.J. Probiotic treatment restores protection against lethal fungal infection lost during amphibian captivity. Proc. R. Soc. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [Green Version]
- Woodhams, D.C.; Alford, R.A.; Antwis, R.E.; Archer, H.; Becker, M.H.; Belden, L.K.; Bell, S.C.; Bletz, M.; Daskin, J.H.; Davis, L.R.; et al. Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology 2015, 96, 595. [Google Scholar] [CrossRef] [Green Version]
- Antwis, R.E.; Harrison, X.A. Probiotic consortia are not uniformly effective against different amphibian chytrid pathogen isolates. Mol. Ecol. 2018, 27, 577–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, X.A.; Sewell, T.; Fisher, M.; Antwis, R.E. Designing Probiotic Therapies with Broad-Spectrum Activity Against a Wildlife Pathogen. Front. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Rashed, Z.E.; Corradino, V.; Catalano, A.; Rovegno, L.; Queirolo, L.; Salvidio, S.; Biggi, E.; Zanotti-Russo, M.; Canesi, L.; et al. Peptides for skin protection and healing in amphibians. Molecules 2019, 24, 347. [Google Scholar] [CrossRef] [Green Version]
- Rollins-Smith, L.A.; Ramsey, J.P.; Pask, J.D.; Reinert, L.K.; Woodhams, D.C. Amphibian immune defenses against chytridiomycosis: Impacts of changing environments. Integr. Comp. Biol. 2011, 51, 552–562. [Google Scholar] [CrossRef]
- Klein, S.L.; Strausberg, R.L.; Wagner, L.; Pontius, J.; Clifton, S.W.; Richardson, P. Genetic and genomic tools for Xenopus research: The NIH Xenopus initiative. Dev. Dyn. 2002, 225, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Vas, J.; Grönwall, C.; Silverman, G. Fundamental roles of the innate-like repertoire of natural antibodies in immune homeostasis. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Roelants, K.; Fry, B.G.; Ye, L.; Stijlemans, B.; Brys, L.; Kok, P.; Clynen, E.; Schoofs, L.; Cornelis, P.; Bossuyt, F. Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [Green Version]
- Woodhams, D.C.; Bigler, L.; Marschang, R. Tolerance of fungal infection in European water frogs exposed to Batrachochytrium dendrobatidis after experimental reduction of innate immune defenses. BMC Vet. Res. 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Daum, J.M.; Davis, L.R.; Bigler, L.; Woodhams, D.C. Hybrid advantage in skin peptide immune defenses of water frogs (Pelophylax esculentus) at risk from emerging pathogens. Infec. Genet. Evol. 2012, 12, 1854–1864. [Google Scholar] [CrossRef]
- Pask, J.D.; Woodhams, D.C.; Rollins-Smith, L.A. The ebb and flow of antimicrobial skin peptides defends northern leopard frogs (Rana pipiens) against chytridiomycosis. Glob. Chang. Biol. 2012, 18, 1231–1238. [Google Scholar] [CrossRef]
- Thekkiniath, J.C.; Zabet-Moghaddam, M.; San Francisco, S.K.; San Francisco, M.J. A novel subtilisin-like serine protease of Batrachochytrium dendrobatidis is induced by thyroid hormone and degrades antimicrobial peptides. Fungal Biol. 2013, 117, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Adaptation to the Land: The Skin of Reptiles in Comparison to That of Amphibians and Endotherm Amniotes. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Cramp, R.L.; McPhee, R.K.; Meyer, E.A.; Ohmer, M.E.; Franklin, C.E. First line of defence: The role of sloughing in the regulation of cutaneous microbes in frogs. Conserv. Physol. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Ohmer, M.E.B.; Cramp, R.L.; White, C.R.; Harlow, P.S.; McFadden, M.S.; Merino-Viteri, A.; Pessier, A.P.; Wu, N.C.; Bishop, P.J.; Franklin, C.E. Phylogenetic investigation of skin sloughing rates in frogs: Relationships with skin characteristics and disease-driven declines. Proc. R. Soc. B Biol. Sci. 2019, 286. [Google Scholar] [CrossRef] [Green Version]
- Ohmer, M.E.B.; Cramp, R.L.; White, C.R.; Franklin, C.E. Skin sloughing rate increases with chytrid fungus infection load in a susceptible amphibian. Funct. Ecol. 2015, 29, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, E.B.; Poorten, T.J.; Settles, M.; Murdoch, G.K. Only skin deep: Shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol. Ecol. 2012, 21, 3110–3120. [Google Scholar] [CrossRef]
- Rosenblum, E.B.; Poorten, T.J.; Settles, M.; Murdoch, G.K.; Robert, J.; Maddox, N.; Eisen, M.B. Genome-wide transcriptional response of Silurana (Xenopus) tropicalis to infection with the deadly chytrid fungus. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Grogan, L.F.; Cashins, S.D.; Skerratt, L.F.; Berger, L.; McFadden, M.S.; Harlow, P.; Hunter, D.A.; Scheele, B.C.; Mulvenna, J. Evolution of resistance to chytridiomycosis is associated with a robust early immune response. Mol. Ecol. 2018, 27, 919–934. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.; Speare, R.; Hyatt, A. Chytrid fungi and amphibian declines: Overview, implications and future directions. In Declines and Disappearances of Australian Frogs; Campbell, A., Ed.; Environment Australia: Canberra, Australia, 1999; pp. 23–33. [Google Scholar]
- Pessier, A.P.; Nichols, D.K.; Longcore, J.E.; Fuller, M.S. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea). J. Vet. Diagn. Investig. 1999, 11, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.K.; Lamirande, E.W.; Pessier, A.P.; Longcore, J.E. Experimental transmission of cutaneous chytridiomycosis in Dendrobatid frogs. J. Wildl. Dis. 2001, 37, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.; Speare, R.; Skerratt, L.F. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis. Aquat. Org. 2005, 68, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.; Whitehorn, P.; Berger, L.; Skerratt, L.F.; Speare, R.; Garland, S.; Webb, R. Defects in host immune function in tree frogs with Chronic Chytridiomycosis. PLoS ONE 2014, 9, e107284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, A.D.; Boyle, D.G.; Olsen, V.; Boyle, D.B.; Berger, L.; Obendorf, D.; Dalton, A.; Kriger, K.; Hero, M.; Hines, H.; et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2007, 73, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Wilber, M.Q.; Langwig, K.E.; Kilpatrick, A.M.; McCallum, H.I.; Briggs, C.J. Integral Projection Models for host–parasite systems with an application to amphibian chytrid fungus. Methods Ecol. Evol. 2016, 7, 1182–1194. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Nie, L.; Cai, S.-Y.; Shao, J.-Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef]
- Plato, A.; Hardison, S.E.; Brown, G.D. Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 2015, 37, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Randow, F.; MacMicking, J.D.; James, L.C. Cellular self-defense: How cell-autonomous immunity protects against pathogens. Science 2013, 340, 701–706. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi. FEMS Yeast Res. 2010, 10, 225–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016, 44, D7–D19. [Google Scholar] [CrossRef] [Green Version]
- Ellison, A.R.; Savage, A.E.; DiRenzo, G.V.; Langhammer, P.; Lips, K.R.; Zamudio, K.R. Fighting a losing battle: Vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 Genes Genome Genet. 2014, 4, 1275–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rooij, P.; Martel, A.; D’Herde, K.; Brutyn, M.; Croubels, S.; Ducatelle, R.; Haesebrouck, F.; Pasmans, F. Germ tube mediated invasion of Batrachochytrium dendrobatidis in Amphibian skin is host dependent. PLoS ONE 2012, 7, e41481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, S.J.; Garner, T.W.J.; Balloux, F.; Ruis, C.; Paszkiewicz, K.H.; Moore, K.; Griffiths, A.G.F. A de novo assembly of the common frog (Rana temporaria) transcriptome and comparison of transcription following exposure to Ranavirus and Batrachochytrium dendrobatidis. PLoS ONE 2015, 10, e0130500. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Shenoy, A.R.; Kumar, P.; Bradfield, C.J.; MacMicking, J.D. IFN-inducible GTPases in host cell defense. Cell Host Microbe 2012, 12, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Pilla-Moffett, D.; Barber, M.F.; Taylor, G.A.; Coers, J. Interferon-Inducible GTPases in Host Resistance, Inflammation and Disease. J. Mol. Biol. 2016, 428, 3495–3513. [Google Scholar] [CrossRef] [Green Version]
- Meunier, E.; Broz, P. Interferon-inducible GTPases in cell autonomous and innate immunity. Cell. Microbiol. 2016, 18, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Brannelly, L.A.; Roberts, A.A.; Skerratt, L.F.; Berger, L. Epidermal cell death in frogs with chytridiomycosis. PeerJ 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Fites, J.S.; Ramsey, J.P.; Holden, W.M.; Collier, S.P.; Sutherland, D.M.; Reinert, L.K.; Gayek, A.S.; Dermody, T.S.; Aune, T.M.; Oswald-Richter, K.; et al. The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 2013, 342, 366–369. [Google Scholar] [CrossRef] [Green Version]
- Woodhams, D.C.; Ardipradja, K.; Alford, R.A.; Marantelli, G.; Reinert, L.K.; Rollins-Smith, L.A. Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim. Conserv. 2007, 10, 409–417. [Google Scholar] [CrossRef]
- Davis, A.K.; Keel, M.K.; Ferreira, A.; Maerz, J.C. Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp. Clin. Pathol. 2010, 19, 49–55. [Google Scholar] [CrossRef]
- Peterson, J.D.; Steffen, J.E.; Reinert, L.K.; Cobine, P.A.; Appel, A.; Rollins-Smith, L.; Mendonça, M.T. Host Stress Response Is Important for the Pathogenesis of the Deadly Amphibian Disease, Chytridiomycosis, in Litoria caerulea. PLoS ONE 2013, 8, e62146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fites, S.J.; Reinert, L.K.; Chappell, T.M.; Rollins-Smith, L.A. Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infect. Immun. 2014, 82, 4698–4706. [Google Scholar] [CrossRef] [Green Version]
- Gantress, J.; Maniero, G.D.; Cohen, N.; Robert, J. Development and characterization of a model system to study amphibian immune responses to iridoviruses. Virology 2003, 311, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Maniero, G.D.; Morales, H.; Gantress, J.; Robert, J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev. Comp. Immunol. 2006, 30, 649–657. [Google Scholar] [CrossRef]
- Sommer, S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front. Zool. 2005, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Thomson, G. How selection shapes variation of the human major histocompatibility complex: A review. Ann. Hum. Genet. 2001, 65, 1–26. [Google Scholar] [CrossRef]
- Kosch, T.A.; Silva, C.N.S.; Brannelly, L.A.; Roberts, A.A.; Lau, Q.; Marantelli, G.; Berger, L.; Skerratt, L.F. Genetic potential for disease resistance in critically endangered amphibians decimated by chytridiomycosis. Anim. Conserv. 2019, 22, 238–250. [Google Scholar] [CrossRef]
- Bataille, A.; Cashins, S.D.; Grogan, L.; Skerratt, L.F.; Hunter, D.; McFadden, M.; Scheele, B.; Brannelly, L.A.; Macris, A.; Harlow, P.S.; et al. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [Green Version]
- Savage, A.E.; Mulder, K.P.; Torres, T.; Wells, S. Lost but not forgotten: MHC genotypes predict overwinter survival despite depauperate MHC diversity in a declining frog. Conserv. Genet. 2017, 19, 309–322. [Google Scholar] [CrossRef]
- Savage, A.E.; Zamudio, K.R. MHC genotypes associate with resistance to a frog-killing fungus. Proc. Natl. Acad. Sci. USA 2011, 108, 16705–16710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stice, M.J.; Briggs, C.J. Immunization is ineffective at preventing infection and mortality due to the amphibian chytrid fungus Batrachochytrium dendrobatidis. J. Wildl. Dis. 2010, 46, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashins, S.D.; Grogan, L.F.; McFadden, M.; Hunter, D.; Harlow, P.S.; Berger, L.; Skerratt, L.F. Prior Infection Does Not Improve Survival against the Amphibian Disease Chytridiomycosis. PLoS ONE 2013, 8, e56747. [Google Scholar] [CrossRef] [Green Version]
- McMahon, T.A.; Sears, B.F.; Venesky, M.D.; Bessler, S.M.; Brown, J.M.; Deutsch, K.; Halstead, N.T.; Lentz, G.; Tenouri, N.; Young, S.; et al. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 2014, 511, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, J.R.; Waddle, A.W.; Rivera, R.; Harrison, D.T.; Ellison, S.; Forrest, M.J.; Vredenburg, V.T.; van Breukelen, F. Batrachochytrium dendrobatidis and the Decline and Survival of the Relict Leopard Frog. EcoHealth 2017, 14, 285–295. [Google Scholar] [CrossRef]
- Grogan, L.F.; Mulvenna, J.; Gummer, J.P.A.; Scheele, B.C.; Berger, L.; Cashins, S.D.; McFadden, M.S.; Harlow, P.; Hunter, D.A.; Trengove, R.D.; et al. Survival, gene and metabolite responses of Litoria verreauxii alpina frogs to fungal disease chytridiomycosis. Sci. Data 2018, 5, 180033. [Google Scholar] [CrossRef] [PubMed]
- Waddle, A.W.; Levy, J.E.; Rivera, R.; van Breukelen, F.; Nash, M.; Jaeger, J.R. Population-Level Resistance to Chytridiomycosis is Life-Stage Dependent in an Imperiled Anuran. EcoHealth 2019, 16, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P. Innate immune memory: Time for adopting a correct terminology. Front. Immunol. 2018, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Brem, F.; Parris, M. Epidermal trauma reduces the impact of Batrachochytrium dendrobatidis in Fowler’s toads (Anaxyrus fowleri). Open Zool. J. 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Eskew, E.A.; Shock, B.C.; Ladouceur, E.E.B.; Keel, K.; Miller, M.R.; Foley, J.E.; Todd, B.D. Gene expression differs in susceptible and resistant amphibians exposed to Batrachochytrium dendrobatidis. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, A.R.; Tunstall, T.; Direnzo, G.V.; Hughey, M.C.; Rebollar, E.A.; Belden, L.K.; Harris, R.N.; Ibáñez, R.; Lips, K.R.; Zamudio, K.R. More than skin deep: Functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biolog. Evol. 2014, 7, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollins-Smith, L.A.; Fites, J.S.; Reinert, L.K.; Shiakolas, A.R.; Umile, T.P.; Minbiole, K.P.C. Immunomodulatory metabolites released by the frog-killing fungus Batrachochytrium dendrobatidis. Infect. Immun. 2015, 83, 4565–4570. [Google Scholar] [CrossRef] [Green Version]
- Rollins-Smith, L.A.; Ruzzini, A.C.; Fites, J.S.; Reinert, L.K.; Hall, E.M.; Joosse, B.A.; Ravikumar, V.I.; Huebner, M.I.; Aka, A.; Kehs, M.H.; et al. Metabolites involved in immune evasion by Batrachochytrium dendrobatidis include the polyamine spermidine. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Raberg, L.; Graham, A.L.; Read, A.F. Decomposing health: Tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. B-Biol. Sci. 2009, 364, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Raberg, L.; Sim, D.; Read, A.F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007, 318, 812–814. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Savage, A.E.; Gratwicke, B.; Hope, K.; Bronikowski, E.; Fleischer, R.C. Sustained immune activation is associated with susceptibility to the amphibian chytrid fungus. Mol. Ecol. 2020, 29, 2889–2903. [Google Scholar] [CrossRef]
- Claytor, S.C.; Gummer, J.P.A.; Grogan, L.F.; Skerratt, L.F.; Webb, R.J.; Brannelly, L.A.; Berger, L.; Roberts, A.A. Susceptibility of frogs to chytridiomycosis correlates with increased levels of immunomodulatory serotonin in the skin. Cell. Microbiol. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Huergo, L.F.; Dixon, R. The Emergence of 2-Oxoglutarate as a Master Regulator Metabolite. Microbiol. Mol. Biol. Rev. 2015, 79, 419–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannelly, L.A.; Martin, G.; Llewelyn, J.; Skerratt, L.F.; Berger, L. Age- and size-dependent resistance to chytridiomycosis in the invasive cane toad Rhinella marina. Dis. Aquat. Org. 2018, 131, 107–120. [Google Scholar] [CrossRef]
- Flies, A.S. Rewilding immunology Integrating comparative immunology can improve human, animal, and ecosystem health. Science 2020, 369, 37–38. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grogan, L.F.; Humphries, J.E.; Robert, J.; Lanctôt, C.M.; Nock, C.J.; Newell, D.A.; McCallum, H.I. Immunological Aspects of Chytridiomycosis. J. Fungi 2020, 6, 234. https://0-doi-org.brum.beds.ac.uk/10.3390/jof6040234
Grogan LF, Humphries JE, Robert J, Lanctôt CM, Nock CJ, Newell DA, McCallum HI. Immunological Aspects of Chytridiomycosis. Journal of Fungi. 2020; 6(4):234. https://0-doi-org.brum.beds.ac.uk/10.3390/jof6040234
Chicago/Turabian StyleGrogan, Laura F., Josephine E. Humphries, Jacques Robert, Chantal M. Lanctôt, Catherine J. Nock, David A. Newell, and Hamish I. McCallum. 2020. "Immunological Aspects of Chytridiomycosis" Journal of Fungi 6, no. 4: 234. https://0-doi-org.brum.beds.ac.uk/10.3390/jof6040234