Comparative Study on Lead and Copper Biosorption Using Three Bioproducts from Edible Mushrooms Residues
Abstract
:1. Introduction
2. Materials and Methods
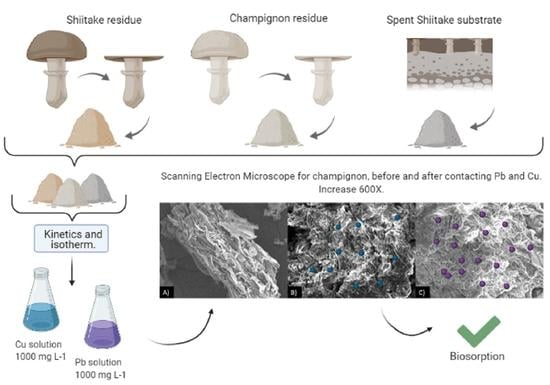
2.1. Sample Preparation
2.2. Removal Efficiency Over Time
2.3. Adsorption Isotherm
2.4. Evaluation of Copper (Cu) and Lead (Pb) in Water
2.5. Biomaterial Morphology
3. Results and Discussion
3.1. Removal Efficiency over Time
3.2. Adsorption Isotherm
3.3. Scanning Electron Microscope (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, C.-H.; Oh, S.J.; Shin, Y.; Han, S.-H.; Nam, I.-H.; So, J.-S. Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol. Eng. 2015, 74, 402–407. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibulla-Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Abalaka, S.E.; Enem, S.I.; Idoko, I.S.; Sani, N.A.; Tenuche, O.Z.; Ejeh, S.A.; Sambo, W.K. Heavy Metals bioaccumulation and health risks with associated histopathological changes in Clarias gariepinus from the Kado Fish Market, Abuja, Nigeria. J. Health Pollut. 2020, 10, 200602. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, G.; He, Y.; Chen, C.; Liu, X.; Li, G.; Gu, Y.; Zhao, Y. Mixed heavy metals removal from wastewater by discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ. Sci. Process. Impacts 2019, 21, 584–592. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Rees, N.; Fuller, R. The Toxic Truth: Children’s Exposure to Lead Pollution Undermines a Generation of Future Potential, 2nd ed.; Unicef and Pure Earth: New York, NY, USA, 2020; Available online: https://www.unicef.org/media/73246/file/The-toxic-truth-children%E2%80%99s-exposure-to-lead-pollution-2020.pdf (accessed on 15 January 2021).
- Meena, R.A.A.; Sathishkumar, P.; Ameen, F.; Yusoff, A.R.M.; Gu, F.L. Heavy metal pollution in immobile and mobile components of lentic ecosystems-a review. Environ. Sci. Pollut. Res. Int. 2017, 25, 4134–4148. [Google Scholar] [CrossRef]
- Chaney, R.L.; Mielke, H.W.; Sterrett, S.B. Speciation, mobility, and bioavailability of soil lead. Proc. Intern. Conf. Lead in Soils: Issues and Guidelines. B.E. Davies & B.G. Wixson (eds.). Environ. Geochem. Health 1989, 11, 105–129. [Google Scholar]
- European Food Safety Authority. Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Do Nascimento, S.N.; Barth, A.; Göethel, G.; Baierle, M.; Charão, M.F.; Brucker, N.; Moro, A.M.; Bubols, G.B.; Sobreira, J.S.; Sauer, E.; et al. Cognitive deficits and ALA-D-inhibition in children exposed to multiple metals. Environ. Res. 2015, 136, 387–395. [Google Scholar] [CrossRef]
- Dórea, J.G. Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children. Environ. Res. 2019, 177, 1–13. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ. Manag. 2019, 65, 131–159. [Google Scholar] [CrossRef] [Green Version]
- U.S. EPA. Regional Screening Levels (RSLs)—Generic Tables November 2020. U.S. EPA: Washington, DC, USA. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 8 May 2021).
- Kalita, J.; Kumar, V.; Misra, U.K.; Bora, H.K. Memory and learning dysfunction following copper toxicity: Biochemical and immunohistochemical basis. Mol. Neurobiol 2017, 55, 3800–3811. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Interaction Profile for Arsenic, Cadmium, Chromium and Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov/interactionprofiles/ip-metals2/ip06.pdf (accessed on 4 May 2021).
- Companhia Ambiental do Estado de São Paulo (CETESB). Cobre. FiT—Ficha de Informação Toxicológica. 2020. Available online: https://cetesb.sp.gov.br/laboratorios/wp-content/uploads/sites/24/2013/11/Cobre.pdf (accessed on 4 May 2021).
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi., A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Squitti, R.; Ghidoni, R.; Simonelli, I.; Ivanova, I.D.; Colabufo, N.A.; Zuin, M.; Benussi, L.; Binetti, G.; Cassetta, E.; Rongioletti, M.; et al. Copper dyshomeostasis in Wilson disease and Alzheimer’s disease as shown by serum and urine copper indicators. J. Trance Elem. Med. Bio. 2018, 45, 181–188. [Google Scholar] [CrossRef]
- Kimmel, C.A.; Garry, M.R.; DeSesso, J.M. Relationship between bent long bones, bent scapulae, and wavy ribs: Malformations or variations? Birth Defects Res. B Dev. Reprod. Toxicol. 2014, 101, 379–392. [Google Scholar] [CrossRef]
- World Health Organization. Water Treatment. Who Seminar Pack for Drinking-Water Quality. 2007. Available online: http://www.who.int/water_sanitation_health/dwq/S12.pdf (accessed on 28 April 2021).
- Bernoth, L.; Firth, I.; Mcallister, P.; Rhodes, S. Biotechnologies for remediation and pollution control in the mining industry. Miner. Metall. Proc. 2000, 17, 105–111. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.; Alba-Elías, F. Bioremediation of wastewater to remove heavy metals using the spent mushroom substrate of Agaricus bisporus. Water 2019, 11, 454. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Nizamuddin, S.; Karri, R.R. Magnetic nanoadsorbents’ potential route for heavy metals removal—A review. Environ. Sci. Pollut. Res. 2020, 27, 24342–24356. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef]
- Kulshreshtha, S. Removal of pollutants using spent mushrooms substrates. Environ. Chem. Lett. 2019, 17, 833–847. [Google Scholar] [CrossRef]
- Lau, K.L.; Tsang, Y.Y.; Chiu, S.W. Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere 2003, 52, 1539–1546. [Google Scholar] [CrossRef]
- Nitschke, J.; Altenbach, H.-J.; Malolepszy, T.; Mölleken, H. A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr. Res. 2011, 346, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Rasoulzadeh, H.; Dehghani, M.H.; Mohammadi, A.S.; Karri, R.R.; Nabizadeh, R.; Nazmara, S.; Kim, K.-H.; Sahu, J.N. Parametric modelling of Pb (II) adsorption onto chitosan-coated Fe3O4 particles through RSM and DE hybrid evolutionary optimization framework. J. Mol. Li. 2019, 297, 111893. [Google Scholar] [CrossRef]
- Ertugay, N.; Bayhan, Y.K. Biosorption of Cr (VI) from aqueous solutions by biomass of Agaricus bisporus. J. Hazard. Mater. 2008, 154, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ertugay, N.; Bayhan, Y.K. The removal of copper (II) ion by using mushroom biomass (Agaricus bisporus) and kinetic modelling. Desalination 2010, 255, 137–142. [Google Scholar] [CrossRef]
- Soldi, E.; Casey, C.; Murphy, B.R.; Hodkinson, T.R. Fungal endophytes for grass-based bioremediation: An endophytic consortium isolated from Agrostis stolonifera stimulates the growth of Festuca arundinacea in lead contaminated soil. J. Fungi. 2020, 6, 254. [Google Scholar] [CrossRef]
- Menk, J.J.; Nascimento, A.I.S.; Leite, F.G.; Oliveira, R.A.; Jozala, A.F.; Oliveira Junior, J.M.; Chaud, M.V.; Grotto, D. Biosorption of pharmaceutical products by mushroom stem waste. Chemosphere 2019, 237, 124515. [Google Scholar] [CrossRef]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Bioaccumulation and biosorption activities of indoor metal-tolerant Penicillium simplicissimum for removal of toxic metals. Int. J. Environ. Res. 2020, 14, 235–242. [Google Scholar] [CrossRef]
- Amar, M.B.; Walha, K.; Salvadó, V. Evaluation of Olive Stones for Cd(II), Cu(II), Pb(II) and Cr(VI) Biosorption from aqueous solution: Equilibrium and kinetics. Int. J. Environ. Res. 2020, 14, 193–204. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Pedron, T.; Segura, F.R.; da Silva, F.F.; de Souza, A.L.; Maltez, H.F.; Batista, B.L. Essential and non-essential elements in Brazilian infant food and other rice-based products frequently consumed by children and celiac population. J. Food Compot. Anal. 2016, 49, 78–86. [Google Scholar] [CrossRef]
- Liu, H.; Guo, S.; Jia, Z.; Han, Y.; He, Q.; Xu, H. Alleviating the toxicity of heavy metals by combined amendments in cultivated bag of Pleurotus cornucopiae. Environ. Sci. Pollut. R 2015, 22, 17182–17191. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.-S.Z.; Amin, N.K.; Abdelwahab, O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 2008, 223, 162–173. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Sheng, F.; Qing, H. Adsorption characteristics of Copper (Ⅱ), Zinc (Ⅱ) and Mercury (Ⅱ) by four kinds of immobilized fungi residues. Ecotox. Environ. Saf. 2018, 147, 357–366. [Google Scholar] [CrossRef]
- Liu, S.H.; Zeng, G.M.; Niu, Q.Y.; Liu, Y.; Zhou, L.; Jiang, L.H.; Tan, X.F.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Dubey, S.P.; Gopal, K.; Sillanpää, M. Strengthening adsorptive amelioration: Isotherm modeling in liquid phase surface complexation of Pb (II) and Cd (II) ions. Desalination 2011, 267, 25–33. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Chen, Y.; Shah, R.N.; Shimizu, K.D. Characterization of molecularly imprinted polymers with the Langmuir−Freundlich isotherm. Anal. Chem. 2001, 73, 4584–4591. [Google Scholar] [CrossRef]
- García-Delgado, C.; Jiménez-Ayuso, N.; Frutos, I.; Gárate, A.; Eymar, E. Cadmium and lead bioavailability and their effects on polycyclic aromatic hydrocarbons biodegradation by spent mushroom substrate. Environ. Sci. Pollut. R 2013, 20, 8690–8699. [Google Scholar] [CrossRef]
- Qiao, X.; Huang, W.; Bian, Y. Effective removal of cadmium ions from a simulated gastrointestinal fluid by Lentinus edodes. Int. J. Environ. Res. Public. Health 2014, 11, 12486–12498. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameters | Stalk of Champignon | Stalk of Shiitake | Substrate of Shiitake |
|---|---|---|---|
| Copper (Cu) | |||
| R2 | 0.889 | 0.546 | 0.970 |
| K (L/mg−1) | 3.21 × 10−5 | 8.82 × 10−5 | 2.6 × 10−4 |
| qmax (mg/g−1) | 31.44 | 22.68 | 14.57 |
| n | 0.56 | 0.43 | 2.04 |
| Lead (Pb) | |||
| R2 | 0.953 | 0.991 | 0.919 |
| K (L/mg−1) | 1.40 × 10−5 | 9.56 × 10−5 | 1.48 × 10−5 |
| qmax (mg/g−1) | 86.74 | 90.18 | 8.79 |
| n | 1.95 | 1.47 | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanho, N.R.C.M.; de Oliveira, R.A.; Batista, B.L.; Freire, B.M.; Lange, C.; Lopes, A.M.; Jozala, A.F.; Grotto, D. Comparative Study on Lead and Copper Biosorption Using Three Bioproducts from Edible Mushrooms Residues. J. Fungi 2021, 7, 441. https://0-doi-org.brum.beds.ac.uk/10.3390/jof7060441
Castanho NRCM, de Oliveira RA, Batista BL, Freire BM, Lange C, Lopes AM, Jozala AF, Grotto D. Comparative Study on Lead and Copper Biosorption Using Three Bioproducts from Edible Mushrooms Residues. Journal of Fungi. 2021; 7(6):441. https://0-doi-org.brum.beds.ac.uk/10.3390/jof7060441
Chicago/Turabian StyleCastanho, Nathália R. C. M., Renan A. de Oliveira, Bruno L. Batista, Bruna M. Freire, Camila Lange, André M. Lopes, Angela F. Jozala, and Denise Grotto. 2021. "Comparative Study on Lead and Copper Biosorption Using Three Bioproducts from Edible Mushrooms Residues" Journal of Fungi 7, no. 6: 441. https://0-doi-org.brum.beds.ac.uk/10.3390/jof7060441