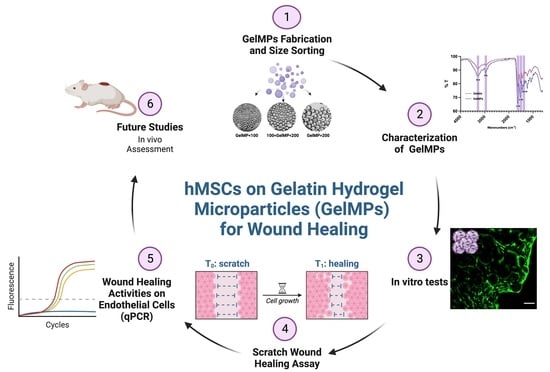
Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Gelatin Hydrogel Microparticles (GelMPs)
2.2. Characterization of Gelatin Hydrogel Microparticles (GelMPs)
2.3. Growth of hMSCs on GelMPs In Vitro
2.4. Effect of Conditioned Media from hMSCs Cultured on GelMPs of Varying Sizes on the Migration of Human Dermal Fibroblasts (HDF)
2.5. Comparison of hMSCs Growth on GelMP < 100 µm versus Encapsulated in GelMA
2.6. Effect of Conditioned Media from hMSCs Cultured on GelMP < 100 µm or in GelMA on the Migration of HDF
2.7. The Effect of Conditioned Media on the Gene Expression of Wound Healing Promoting Factor: PDGF
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Gelatin Hydrogel Microparticles (GelMPs)
4.2. Determination of Degree of Cross-Linking in GelMPs
4.3. ATR-FTIR Analysis
4.4. Determination of Swelling Capacity of GelMPs
4.5. In Situ Degradation of GelMPs in PBS
4.6. Culturing of hMSCs on GelMPs or in GelMA
4.7. AlamarBlue Assay
4.8. Live Staining
4.9. DNA Quantification of hMSC
4.10. Fluorescence Staining of hMSC-Laden GelMPs or GelMA
4.11. Conditioned Media for Migration Assay
4.12. Scratch Wound Migration Assay
4.13. qPCR Analysis
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, T.; Hu, M.S.; Marshall, C.D.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Scarless wound healing: Finding the right cells and signals. Cell Tissue Res. 2016, 365, 483–493. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Kosaric, N.; Kiwanuka, H.; Gurtner, G.C. Stem cell therapies for wound healing. Expert Opin. Biol. Ther. 2019, 19, 575–585. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Protzman, N.M.; Mao, Y.; Long, D.; Sivalenka, R.; Gosiewska, A.; Hariri, R.J.; Brigido, S.A. Placental-Derived Biomaterials and Their Application to Wound Healing: A Review. Bioengineering 2023, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119, Erratum in Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, Q.; Sun, X. Comprehensive Analysis of Cell Therapy on Chronic Skin Wound Healing: A Meta-Analysis. Hum. Gene Ther. 2021, 32, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Pizzolo, G.; Aprili, G.; Franchini, M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone 2006, 39, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Mao, J.J. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol. Bioeng. 2005, 91, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Rahbaran, M.; Zekiy, A.O.; Bahramali, M.; Jahangir, M.; Mardasi, M.; Sakhaei, D.; Thangavelu, L.; Shomali, N.; Zamani, M.; Mohammadi, A.; et al. Therapeutic utility of mesenchymal stromal cell (MSC)-based approaches in chronic neurodegeneration: A glimpse into underlying mechanisms, current status, and prospects. Cell. Mol. Biol. Lett. 2022, 27, 56. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733. [Google Scholar] [CrossRef]
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: A promising frontier. Eur. J. Cell Biol. 2020, 99, 151097. [Google Scholar] [CrossRef]
- Tavakoli, S.; Ghaderi Jafarbeigloo, H.R.; Shariati, A.; Jahangiryan, A.; Jadidi, F.; Jadidi Kouhbanani, M.A.; Hassanzadeh, A.; Zamani, M.; Javidi, K.; Naimi, A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J. Cell. Physiol. 2020, 235, 9185–9210. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.P.; Ni, A.G.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Daneste, H.; Mohammadzadeh Boukani, L.; Ramezani, N.; Asadi, F.; Zaidan, H.K.; Sadeghzade, A.; Ehsannia, M.; Azarashk, A.; Gholizadeh, N. Combination therapy along with mesenchymal stem cells in wound healing; the state of the art. Adv. Med. Sci. 2023, 68, 441–449. [Google Scholar] [CrossRef]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of Allogeneic Adipose-Derived Stem Cell–Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Shahror, R.A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI. Int. J. Mol. Sci. 2020, 21, 4051. [Google Scholar] [CrossRef]
- Sylakowski, K.; Bradshaw, A.; Wells, A. Mesenchymal Stem Cell/Multipotent Stromal Cell Augmentation of Wound Healing. Am. J. Pathol. 2020, 190, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ding, X.M.; Xue, W.J.; Zheng, J.; Tian, X.H.; Li, Y.; Wang, X.H.; Song, H.J.; Liu, H.; Luo, X.H. A new scaffold containing small intestinal submucosa and mesenchymal stem cells improves pancreatic islet function and survival in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.U.; Berthiaume, F.; Dardik, A.; Hsia, H.C. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Tan, F.; Yin, W.; Li, Z. Umbilical cord derived mesenchymal stem cell-GelMA microspheres for accelerated wound healing. Biomed. Mater. 2022, 18, 015019. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Nafea, E.H.; Marson, A.; Poole-Warren, L.A.; Martens, P.J. Immunoisolating semi-permeable membranes for cell encapsulation: Focus on hydrogels. J. Control. Release 2011, 154, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xia, X.; Zhang, K.; Rai, A.; Li, Z.; Zhao, P.; Wei, K.; Zou, L.; Yang, B.; Wong, W.-K.; et al. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci. Transl. Med. 2020, 12, eaba8014. [Google Scholar] [CrossRef]
- Dong, Y.; Rodrigues, M.; Kwon, S.H.; Li, X.; Brett, E.A.; Elvassore, N.; Wang, W.; Gurtner, G.C. Acceleration of Diabetic Wound Regeneration using an In Situ-Formed Stem-Cell-Based Skin Substitute. Adv. Healthc. Mater. 2018, 7, e1800432. [Google Scholar] [CrossRef]
- Dong, Y.X.; Sigen, A.; Rodrigues, M.; Li, X.L.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.S.; Wang, W.X.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606619. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Y.; Lee, L.; Song, T.; Zhou, J.; Yan, L.; Li, Y. Adaptive Gelatin Microspheres Enhanced Stem Cell Delivery and Integration With Diabetic Wounds to Activate Skin Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 813805. [Google Scholar] [CrossRef]
- Chen, R.; Curran, S.J.; Curran, J.M.; Hunt, J.A. The use of poly(l-lactide) and RGD modified microspheres as cell carriers in a flow intermittency bioreactor for tissue engineering cartilage. Biomaterials 2006, 27, 4453–4460. [Google Scholar] [CrossRef]
- Bektas, C.; Mao, Y. Hydrogel Microparticles for Bone Regeneration. Gels 2024, 10, 28. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Kang, J.I.; Park, K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-Based Seamless Scaffolds Containing Macroscopic Gradients of Encapsulated Factors for Tissue Engineering. Tissue Eng. Part C Methods 2008, 14, 299–309. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Kodali, A.; Lim, T.C.; Leong, D.T.; Tong, Y.W. Cell-Microsphere Constructs Formed with Human Adipose-Derived Stem Cells and Gelatin Microspheres Promotes Stemness, Differentiation, and Controlled Pro-Angiogenic Potential. Macromol. Biosci. 2014, 14, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Baipaywad, P.; Jeong, Y.; Park, H. Incorporation of gelatin microparticles on the formation of adipose-derived stem cell spheroids. Int. J. Biol. Macromol. 2018, 110, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Xue, L.; Wang, G.; Li, X.; Chen, J.; Xu, R.; Xu, T. A controllable gelatin-based microcarriers fabrication system for the whole procedures of MSCs amplification and tissue engineering. Regen. Biomater. 2023, 10, rbad068. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Makino, K.; Tabata, Y. Influence of shaking culture on the biological functions of cell aggregates incorporating gelatin hydrogel microspheres. J. Biosci. Bioeng. 2019, 128, 606–612. [Google Scholar] [CrossRef]
- Fan, C.; Zhan, S.-H.; Dong, Z.-X.; Yang, W.; Deng, W.-S.; Liu, X.; Wang, D.-A.; Sun, P. Cross-linked gelatin microsphere-based scaffolds as a delivery vehicle of MC3T3-E1 cells: And evaluation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 108, 110399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, A.; Chen, C.; Tang, J.; Fan, H.; Sun, J.; Fan, H. An efficient two-step preparation of photocrosslinked gelatin microspheres as cell carriers to support MC3T3-E1 cells osteogenic performance. Colloids Surf. B Biointerfaces 2020, 188, 110798. [Google Scholar] [CrossRef]
- Dong, Z.; Fan, C.; Deng, W.; Sun, P. Porous gelatin microsphere-based scaffolds containing MC3T3-E1 cells and calcitriol for the repair of skull defect. Biomater. Adv. 2022, 138, 212964. [Google Scholar] [CrossRef]
- Tajima, S.; Tabata, Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J. Tissue Eng. Regen. Med. 2013, 7, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Chowdhury, S.R.; Fauzi, M.B.; Rani, R.A.; Yahaya, N.H.M.; Tabata, Y.; Hiraoka, Y.; Idrus, R.B.H.; Hwei, N.M. 3D Culture of MSCs on a Gelatin Microsphere in a Dynamic Culture System Enhances Chondrogenesis. Int. J. Mol. Sci. 2020, 21, 2688. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, C.; Li, J.; Bi, L.; Qin, L.; Wu, H.; Hu, Y. Gelatin microspheres containing TGF-β3 enhance the chondrogenesis of mesenchymal stem cells in modified pellet culture. Biomacromolecules 2008, 9, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Tzouanas, S.N.; Ekenseair, A.K.; Kasper, F.K.; Mikos, A.G. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tabata, Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; Cooke, M.T.; Saeed, R.; Kinney, M.A.; Fridley, K.M.; McDevitt, T.C. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J. Mech. Behav. Biomed. Mater. 2012, 11, 63–71. [Google Scholar] [CrossRef]
- Kimura, Y.; Ozeki, M.; Inamoto, T.; Tabata, Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials 2003, 24, 2513–2521. [Google Scholar] [CrossRef]
- Feyen, D.A.M.; Gaetani, R.; Deddens, J.; van Keulen, D.; van Opbergen, C.; Poldervaart, M.; Alblas, J.; Chamuleau, S.; van Laake, L.W.; Doevendans, P.A.; et al. Gelatin Microspheres as Vehicle for Cardiac Progenitor Cells Delivery to the Myocardium. Adv. Health Mater. 2016, 5, 1071–1079. [Google Scholar] [CrossRef]
- Bin Sulaiman, S.; Idrus, R.B.H.; Hwei, N.M. Gelatin Microsphere for Cartilage Tissue Engineering: Current and Future Strategies. Polymers 2020, 12, 2404. [Google Scholar] [CrossRef]
- Gelli, R.; Mugnaini, G.; Bolognesi, T.; Bonini, M. Cross-linked Porous Gelatin Microparticles with Tunable Shape, Size, and Porosity. Langmuir 2021, 37, 12781–12789. [Google Scholar] [CrossRef]
- Chang, S.; Finklea, F.; Williams, B.; Hammons, H.; Hodge, A.; Scott, S.; Lipke, E. Emulsion-based encapsulation of pluripotent stem cells in hydrogel microspheres for cardiac differentiation. Biotechnol. Prog. 2020, 36, e2986. [Google Scholar] [CrossRef]
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008, 4, 1126–1138. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative Reaction Mechanism for the Cross-Linking of Gelatin with Glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Bajpai, J.; Bajpai, A.K. Easy fabrication and characterization of gelatin nanocarriers and in vitro investigation of swelling controlled release dynamics of paclitaxel. Polym. Bull. 2018, 75, 4691–4711. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Arsalani, N.; Ghorbani, M.; Hamishehkar, H. Development of biocompatible fluorescent gelatin nanocarriers for cell imaging and anticancer drug targeting. J. Mater. Sci. 2018, 53, 10679–10691. [Google Scholar] [CrossRef]
- Bahoor, A.; Ahmadi, R.; Heydari, M.; Bagheri, M.; Behnamghader, A. Synthesis and evaluation of cross-linked gelatin nanoparticles for controlled release of an anti-diabetic drug: Gliclazide. Inorg. Chem. Commun. 2023, 154, 110856. [Google Scholar] [CrossRef]
- Zatorski, J.M.; Montalbine, A.N.; Ortiz-Cárdenas, J.E.; Pompano, R.R. Quantification of fractional and absolute functionalization of gelatin hydrogels by optimized ninhydrin assay and 1H NMR. Anal. Bioanal. Chem. 2020, 412, 6211–6220. [Google Scholar] [CrossRef] [PubMed]
- Leane, M.; Nankervis, R.; Smith, A.; Illum, L. Use of the ninhydrin assay to measure the release of chitosan from oral solid dosage forms. Int. J. Pharm. 2004, 271, 241–249. [Google Scholar] [CrossRef]
- Graziola, F.; Candido, T.M.; de Oliveira, C.A.; Peres, D.D.; Issa, M.G.; Mota, J.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; et al. Gelatin-based microspheres crosslinked with glutaraldehyde and rutin oriented to cosmetics. Braz. J. Pharm. Sci. 2016, 52, 603–612. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Adhirajan, N.; Shanmugasundaram, N.; Shanmuganathan, S.; Babu, M. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur. J. Pharm. Sci. 2009, 36, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Correia, D.; Padrão, J.; Rodrigues, L.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Thermal and hydrolytic degradation of electrospun fish gelatin membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef]
- Serban, M.A.; Knight, T.; Payne, R.G.; Basu, J.; Rivera, E.A.; Robbins, N.; McCoy, D.; Halberstadt, C.; Jain, D.; Bertram, T.A. Cross-linked gelatin microspheres with continuously tunable degradation profiles for renal tissue regeneration. Biotechnol. Appl. Biochem. 2014, 61, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and Enzymatically Dual Cross-Linked Oxidized Alginate Gelatin Hydrogels with Tunable Stiffness and Degradation Behavior for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Zhang, F.; Peng, X. In-Vitro Degradation and Cytotoxicity of Gelatin/Chitosan Microspheres for Drug Controlled Release. J. Macromol. Sci. Part A 2014, 51, 646–652. [Google Scholar] [CrossRef]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J.P. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef]
- Mohanty, C.; Pradhan, J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater. Sci. Eng. C 2020, 111, 110751. [Google Scholar] [CrossRef] [PubMed]
- Badiavas, E.V.; Falanga, V. Treatment of chronic wounds with bone marrow-derived cells. Arch. Dermatol. 2003, 139, 510–516. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, K.; Gao, X.; Liu, Y.B.; Dulchavsky, D.S.; Kwon, D.; Arbab, A.S.; Bansal, M.; Li, Y.; Chopp, M.; Dulchavsky, S.A.; et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Noverina, R.; Widowati, W.; Ayuningtyas, W.; Kurniawan, D.; Afifah, E.; Laksmitawati, D.R.; Rinendyaputri, R.; Rilianawati, R.; Faried, A.; Bachtiar, I.; et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clin. Nutr. Exp. 2019, 24, 34–44. [Google Scholar] [CrossRef]
- Bussche, L.; Harman, R.M.; Syracuse, B.A.; Plante, E.L.; Lu, Y.C.; Curtis, T.M.; Ma, M.L.; Van de Walle, G.R. Microencapsulated equine mesenchymal stromal cells promote cutaneous wound healing in vitro. Stem Cell Res. Ther. 2015, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.-A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. Histochem. J. 2020, 51, 251–263. [Google Scholar] [CrossRef]
- Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603, Erratum in Int. J. Nanomed. 2022, 17, 3601–3602. [Google Scholar] [CrossRef]
- Zhu, M.X.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Zhang, W.; Mao, Y.; Wu, X.; Kohn, J. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering 2022, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, H.; Lee, B.H.; Tan, L.P. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, R.; Boggio, P.; Pertusi, G.; Graziola, F.; Bozzo, C.; Colombo, E. Cytokines, chemokines and growth factors “in vitro” and wound healing. J. Investig. Dermatol. 2010, 130, S61. [Google Scholar]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Mikos, A.G.; Jansen, J.A. Porcine Gelatin Microsphere/Calcium Phosphate Cement Composites: A Degradation Study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 555–561. [Google Scholar] [CrossRef]
- Link, D.P.; van den Dolder, J.; van den Beucken, J.J.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-β1 loaded gelatin microparticles. Biomaterials 2008, 29, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Felekori, M.; Khakbiz, M.; Nezafati, N.; Mohammadi, J.; Eslaminejad, M.B. Comparative analysis and properties evaluation of gelatin microspheres crosslinked with glutaraldehyde and 3-glycidoxypropyltrimethoxysilane as drug delivery systems for the antibiotic vancomycin. Int. J. Pharm. 2019, 557, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, Y.; Yamada, M.; Utoh, R.; Seki, M. Expansion of Chinese hamster ovary cells via a loose cluster-assisted suspension culture using cell-sized gelatin microcarriers. J. Biosci. Bioeng. 2023, 135, 417–422. [Google Scholar] [CrossRef]
- Mao, Y.; John, N.; Protzman, N.M.; Kuehn, A.; Long, D.; Sivalenka, R.; Junka, R.A.; Gosiewska, A.; Hariri, R.J.; Brigido, S.A. A decellularized flowable placental connective tissue matrix supports cellular functions of human tenocytes in vitro. J. Exp. Orthop. 2022, 9, 69. [Google Scholar] [CrossRef]
- Mao, Y.; Block, T.; Singh-Varma, A.; Sheldrake, A.; Leeth, R.; Griffey, S.; Kohn, J. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomater. 2019, 85, 75–83. [Google Scholar] [CrossRef] [PubMed]







| Primers | ID |
|---|---|
| Quanti-tect GAPDH | QT01192646 |
| Quanti-tect PDGFB | QT00001260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozhava, D.; Bektas, C.; Lee, K.; Jackson, A.; Mao, Y. Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities. Gels 2024, 10, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/gels10020097
Ozhava D, Bektas C, Lee K, Jackson A, Mao Y. Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities. Gels. 2024; 10(2):97. https://0-doi-org.brum.beds.ac.uk/10.3390/gels10020097
Chicago/Turabian StyleOzhava, Derya, Cemile Bektas, Kathleen Lee, Anisha Jackson, and Yong Mao. 2024. "Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities" Gels 10, no. 2: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/gels10020097