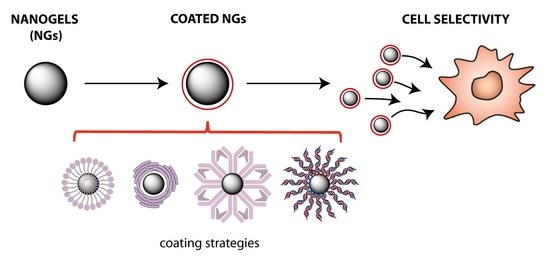
Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery
Abstract
:1. Introduction
2. Nanoparticles and Nanogels
2.1. Definition and Properties
2.2. Synthesis and Formation Methods
2.3. Polymer Functionalization for Nanoparticles Synthesis
- The activation of esters under mild conditions to form amide bonds. This technique represents one of the most interesting strategies for polymer functionalization: esters [40] are very flexible for different kinds of functionalization while amides are very versatile linkages in organic chemistry thanks to their stability in chemical environments and compatibility with different functionalities.
- Click chemistry, which allows the introduction of compatible click functional groups in pre-existing molecules that are able to activate molecules and polymers [41]. This process is usually performed with copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC process), but it can also be realized with a copper-free strain promoted azide–alkyne process to overcome the cytotoxicity of the CuAAC reaction [42].This technique is stereospecific and generates non-toxic byproducts that can be easily removed. Moreover, functionalization with azide and alkyne groups offers the possibility to link very different molecules and improve material properties and compatibility.
- Thiol chemistry represents another important process in this field thanks to the great available functional thiols and reactions in which they can participate [43,44]. They have radical- and light-mediated reactivity with carbon–carbon double bonds, and this allows the advantages of click reactions to be combined with those of photoinitiated reactions [45]. In this way, it is possible to obtain quantitative yields, high reaction rates, and easy product recovery. At the same time, the method based on electrophile and nucleophile interactions allows operation in mild reaction conditions, has great compatibility with different functional groups, high conversion, and very good possibilities of application in different fields such as biomedicine.
- The addition of alcohols, amines and thiols to isocyanates [46] represents a possible effective strategy in polymer functionalization thanks to fast reaction kinetics, the stability of isocyanates toward radicals, and the good yields. However, their application is limited by the toxicity of isocyanate and the instability of final mixtures containing isocyanate and polymer mixtures.
- Imine [47] and oxime [48,49] linkages have an important role in the field of macromolecular modification reactions. They allow the bond reversibility in the case of imine linkage thanks to the equilibrium of this molecule and in the case of oxime under aqueous acid conditions. This functionalization method can be tuned according to the desired application: the obtained linkage can be hydrolytically stable or unstable according to the need, in order to preserve the functionalization or to release the grafted molecule.
- Ring-opening reactions are a classical and versatile method in polymer science [50]. They allow the ring of strained heterocycles to be opened and for desired heteroatoms to be introduced on the polymer backbone [51,52]. The most commonly employed functional groups in this kind of synthesis are the epoxides, but in recent years ring-opening modifications involving aziridines and azlactones have been reported.
- Multicomponent reactions (MCRs) [53] are an upcoming synthesis methodology which are of great interest because of their atom economy. Their advantage lie in their ability to introduce a high degree of functional complexity in a single modification step [54]. These kinds of reactions include isocyanide-based MCRs, non-isocyanide-based MCRs, and MCRs catalyzed by organometallic species.
3. Targeted Drug Delivery: Selectivity of the Delivery Process and Its Applications
4. Available Strategies to Coat Nanoparticles
4.1. Polymers
4.2. An Organic Solution: Cell Membrane Coating
4.3. Proteins and Antibodies: A Very Promising Possibility
4.4. Novel Strategy: Hybridized DNA Structure
5. Conclusions
Conflicts of Interest
References
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Chapter 11 Polymeric Nanoparticles for Drug Delivery. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 163–175. [Google Scholar]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, Y.; Liu, S.; Wang, Y.; Liu, Q. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm. Sin. B 2019, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kohrs, N.J.; Liyanage, T.; Venkatesan, N.; Najarzadeh, A.; Puleo, D.A.; States, U. Drug Delivery Systems and Controlled Release. Biomed. Sci. 2019, 2, 316–329. [Google Scholar]
- Kumar, B.; Jajodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 127–133. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Teresa, M. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Gurny, R.; Peppas, N.A.; Harrington, D.D.; Banker, G.S. Development of Biodegradable and Injectable Latices for Controlled release of Potent Drugs. Drug Dev. Ind. Pharm. 1981, 7, 1–25. [Google Scholar] [CrossRef]
- Gharge, V.G.; Bhandare, P.S. Formulation and Evaluation of Microencapsulated Glimepiride Produced by the Emulsion—Solvent Evaporation Method. PharmaTutor 2018, 6, 27–30. [Google Scholar] [CrossRef]
- Gharieh, A.; Khoee, S.; Mahdavian, A.R. Emulsion and Miniemulsion Techniques in Preparation of Polymer Nanoparticles with Versatile Characteristics. Adv. Colloid Interface Sci. 2019, 269, 152–186. [Google Scholar] [CrossRef]
- Nguyen, C.A.; Konan-Kouakou, Y.N.; Allémann, E.; Doelker, E.; Quintanar-Guerrero, D.; Fessi, H.; Gurny, R. Preparation of Surfactant-free Nanoparticles of Methacrylic Acid Copolymers Used for Film Coating. AAPS PharmSciTech 2006, 7, E56–E60. [Google Scholar] [CrossRef] [Green Version]
- Mainardes, R.M.; Evangelista, R.C. Praziquantel-loaded PLGA nanoparticles: Preparation and characterization. J. Microencapsul. 2005, 22, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, M.; Kumar, B.P. Role of Nanoparticles in Drug Delivery System. Int. J. Res. Pharm. Biomed. Sci. 2010, 1, 41–66. [Google Scholar]
- Nagal, A.; Singla, R.K. Nanoparticles in Different Delivery Systems: A Brief Review. Indo Global J. Pharm. Sci. 2013, 3, 96–106. [Google Scholar]
- de Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. BBA Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Dong, P.; Rakesh, K.P.; Manukumar, H.M.; Hussein, Y.; Mohammed, E. Innovative nano-carriers in anticancer drug delivery—A comprehensive review. Bioorg. Chem. 2019, 85, 325–336. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S. A Review on Nanoparticles: Their Synthesis and Type. Res. J. Recent Sci. 2015, 4, 9–11. [Google Scholar]
- Christian, P.; Baalousha, M.; von der Kammer, F.; Hofmann, T. Nanoparticles: Structure, Properties, Preparation and Behaviour in Environmental Media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Zhang, W.; Wang, H.P. A method for the preparation of stable dispersion of zero-valent iron nanoparticles. Colloids Surfaces A Physicochem. Eng. Aspects 2007, 308, 60–66. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Manirujjaman, M.; Imran-Ul-Haque, M.A.; Sharmin, S. An Overview of Nanogel Drug Delivery System. J. Appl. Pharm. Sci. 2013, 3, 95–105. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology (the “Gold Book”); Blackwell Scientific Publications: Oxford, UK, 2014; Volume 1801, p. 7521. [Google Scholar]
- Cho, H.; Jammalamadaka, U.; Tappa, K. Nanogels for Pharmaceutical and Biomedical Applications and Their Fabrication Using 3D Print Technologies. Materials 2018, 11, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.A.; Patel, J.K. Nanogel as Controlled Drug Delivery System. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 37–41. [Google Scholar]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 5. [Google Scholar]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large ph Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S. Sol-Gel Synthesis and Characterization of Ag3(2+x)AlxTi4-xO11+δ (0.0≤x≤1.0) Nanoparticles. RSC Adv. 2016, 6, 6336–6341. [Google Scholar] [CrossRef]
- Mauri, E.; Perale, G.; Rossi, F. Nanogel Functionalization: A Versatile Approach To Meet the Challenges of Drug and Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 6525–6541. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.; Almutairi, A. Nanogels as imaging agents for modalities spanning the electromagnetic spectrum. Mater. Horiz. 2016, 3, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Ik, D.; Myung, J. Biopolymer-based microgels/nanogels for drug delivery applications. Progress Polym. Sci. 2009, 34, 1261–1282. [Google Scholar]
- Takeuchi, T.; Kitayama, Y.; Sasao, R.; Yamada, T.; Toh, K.; Matsumoto, Y.; Kataoka, K. Molecularly Imprinted Nanogels Acquire Stealth In Situ by Cloaking Themselves with Native Dysopsonic Proteins. Angew. Chem. Int. Ed. 2017, 56, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Ulanski, P.; Rosiak, J.M. Encyclopedia of Nanoscience and Nanotechnology. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume VII, pp. 845–871. ISBN 1-58883-001-2. [Google Scholar]
- Bultema, L.A.; Huang, X.; Brauer, D.D.; Theato, P. (Chapter 2) Polymer Functionalization. In Polymer Functionalization Textbook; Bultema, L.A., Huang, X., Brauer, D.D., Theato, P., Eds.; Springer International Publishing: Basel, Switzerland, 2018. [Google Scholar] [CrossRef]
- Bhatia, S. (Chapter 3) Natural Polymers vs Synthetic Polymers. In Natural Polymer Drug Delivery Systems; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-kowollik, C. 50th Anniversary Perspective: Polymer Functionalization. ACS Macromol. 2017, 50, 515–5252. [Google Scholar] [CrossRef]
- Neuman, R.C. (Chapter 15) Carbonyl Compounds: Esters, Amides, and Related Molecules. In Organic Chemistry Textbook by Robert Neuman. Available online: https://chem.ucr.edu/curricular-materials/textbook (accessed on 16 December 2019).
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical—Biology Applications. ACS Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Poonthiyil, V.; Lindhorst, T.K.; Golovko, V.B.; Fairbanks, A.J. Recent applications of click chemistry for the functionalization of gold nanoparticles and their conversion to glyco-gold nanoparticles. Bailstein J. Org. Chem. 2018, 14, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, T.; Finne-Wistrand, A. Synthetic Approaches to Combine the Versatility of the Thiol Chemistry with the Degradability of Aliphatic Polyesters. Polym. Rev. 2019, 60, 86–113. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-yne ‘click’/coupling chemistry and recent applications in polymer and materials synthesis and modif cation. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Yin, C.; Huo, F.; Zhang, J.; Martínez-Máñez, R.; Yang, Y.; Lv, H.; Li, S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 14, 6032–6059. [Google Scholar] [CrossRef]
- Gabriel, L.P.; Zavaglia, C.A.C.; Jardini, A.L.; Dias, C.G.B.T.; Filho, R.M. Isocyanates as Precursors to Biomedical Polyurethanes. Chem. Eng. Trans. 2014, 38, 253–258. [Google Scholar]
- Azuma, Y.; Terashima, T.; Sawamoto, M. Precision Synthesis of Imine-Functionalized Reversible Microgel Star Polymers via Dynamic Covalent Cross-Linking of Hydrogen-Bonding Block Copolymer Micelles. Macromolecules 2017, 50, 587–596. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-Involving Synthesis and Reactions of Oximes. Chem. Rev. 2017, 117, 13039–13122. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; Mukherjee, S.; Costanzo, P.J.; Sumerlin, B.S. Modular oxime functionalization of well-defined alkoxyamine-containing polymers. Polym. Chem. 2012, 3, 1758–1762. [Google Scholar] [CrossRef]
- Acham, V.R.; Dongare, M.K.; Kemnitz, E.; Umbarkar, S.B. An epoxide ring-opening reaction by using sol e gel- synthesized palladium supported on a strontium hydroxyl fluoride catalyst. C. R. Chimie 2016, 19, 1237–1246. [Google Scholar] [CrossRef]
- Dechy-cabaret, O.; Martin-vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Lesma, G.; Bassanini, I.; Bortolozzi, R.; Colletto, C.; Bai, R.; Hamel, E.; Meneghetti, F.; Rainoldi, G.; Stucchi, M.; Sacchetti, A.; et al. Complementary isonitrile-based multicomponent reactions for the synthesis of diversified cytotoxic hemiasterlin analogues. Org. Biomol. Chem. 2015, 13, 11633–11644. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Kanu, B.G.; Poudel, B.K.; Choi, H.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2016, 7, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, O.; Zhu, Y.; Kairemo, K. Targeted Liposomal Drug Delivery in Cancer. Curr. Pharm. Des. 2005, 10, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Ambade, A.V.; Savariar, E.N.; Thayumanavan, S. Dendrimeric micelles for controlled drug release and targeted delivery. Mol. Pharm. 2005, 2, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauri, E.; Cappella, F.; Masi, M.; Rossi, F. PEGylation influences drug delivery from nanogels. J. Drug Deliv. Sci. Technol. 2018, 46, 87–92. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Ma, Y.; Zhang, Z.; Che, H.; Mu, J.; Zhang, X.; Zhang, Z. Synthesis and characterization of polymer-coated manganese ferrite nanoparticles as controlled drug delivery. Appl. Surf. Sci. 2018, 428, 258–263. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, F.; Li, X.; Li, J.; Ma, Y.; Mu, J.; Zhang, Z.; Che, H.; Zhang, X. Controlled synthesis of L-cysteine coated cobalt ferrite nanoparticles for drug delivery. Ceram. Int. 2018, 44, 13588–13594. [Google Scholar] [CrossRef]
- Kundu, P.; Das, S.; Chattopadhyay, N. Managing efficacy and toxicity of drugs: Targeted delivery and excretion. Int. J. Pharm. 2019, 565, 378–390. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive ‘Smart’ Drug Delivery and Tumor Targeting. Trends Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics Across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Gorain, B.; Pandey, M.; Kaur, R.; Kesharwani, P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int. J. Pharm. 2019, 565, 509–522. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couvreur, P.; Puisieux, F. Nano- and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993, 10, 141–162. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grobmyer, S.R.; Moudgil, B.M. Cancer Nanotechnology. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; p. 624. ISBN 978-1-60761-609-2. [Google Scholar]
- Geldenhuys, W.J.; Khayat, M.T.; Yun, J.; Nayeem, M.A. Drug Delivery and Nanoformulations for the Cardiovascular System. Res. Rev. Drug Deliv. 2017, 1, 32–40. [Google Scholar] [PubMed]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2014, 14, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.C.; Crabbe, D.; Krynska, B.; Ansari, R.; Kiani, M.F. Aiming for the heart: Targeted delivery of drugs to diseased cardiac tissue. Expert Opin. Drug Deliv. 2008, 5, 459–470. [Google Scholar] [CrossRef]
- Dikpati, A.; Madgulkar, A.R.; Kshirsagar, S.J.; Bhakear, M.R.; Chahal, A.S. Targeted Drug Delivery to CNS using Nanoparticles. J. Adv. Pharm. Sci. 2012, 2, 179–191. [Google Scholar]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Zhang, Q.; Deng, Y.; Li, X.; Peng, L.; Zuo, X.; Piao, M.; Kuang, X.; Sheng, S.; et al. Recent advances in polymer-based drug delivery systems for local anesthetics. Acta Biomater. 2019, 15, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ramirez, L.G.; Nuno-Donlucas, S.M.; Cesteros, L.C.; Katime, I. Smart copolymeric nanohydrogels: Synthesis, characterization and properties. Mater. Chem. Phys. 2008, 112, 1088–1092. [Google Scholar] [CrossRef]
- Mauri, E.; Veglianese, P.; Papa, S.; Mariani, A.; de Paola, M.; Rigamonti, R.; Chincarini, G.M.F.; Rimondo, S.; Sacchetti, A.; Rossi, F. Chemoselective functionalization of nanogels for microglia treatment. Eur. Polym. J. 2017, 94, 143–151. [Google Scholar] [CrossRef]
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Bio-interactions and risks of enginnered nanoparticles. Environ. Res. 2019, 172, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Lima-Tenório, M.K.; Gómez, E.A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef]
- Tietze, R.; Zalonga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pottler, M.; Durr, S.; Alexiou, C. Magnetic Nanoparticle-based Drug Delivery for Cancer Therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef]
- Lim, B.; Tighe, E.C.; Kong, S.D. The Use of Magnetic Targeting for Drug Delivery into Cardiac Myocytes. J. Magn. Magn. Mater. 2018, 473, 21–25. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2013, 19, 474–481. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.; Udego, I.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials. 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane–Camouflaged Nanoparticles for Cancer Phototherapy. Small 2018, 15, 1804105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, L.; Cai, B.; Bu, L.; Liao, Q.; Guo, S.; Zhao, X.; Dong, W.; Liu, W. Micro fluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Sun, Y.; Su, J.; Liu, G.; Chen, J.; Zhang, X.; Zhang, R.; Jiang, M.; Qiu, M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017, 96, 115–128. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I. Cell Membrane-Camouflaged Nanoparticles: A Promising Biomimetic Strategy for Cancer Theragnostics. Polymers 2018, 10, 983. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Mao, H.; Wang, Y.A.; Cao, Z.; Peng, X.; Wang, X.; Duan, H.; Ni, C.; Yuan, Q.; Adams, G.; et al. Single Chain Epidermal Growth Factor Receptor Antibody Conjugated Nanoparticles for in vivo Tumor Targeting and Imaging. Small-J. 2009, 5, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Kyriakopoulou, K.; Kefali, E.; Piperigkou, Z.; Bassiony, H.; Karamanos, N.K. Advances in targeting epidermal growth factor receptor signaling pathway in mammary cancer. Cell. Signal. 2018, 51, 99–109. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.Y.; Kim, S.H.; Park, K.M.; Hwang, B.H. His-tagged protein immobilization on cationic ferrite magnetic nanoparticles. Korean J. Chem. Eng. 2018, 35, 1297–1302. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, H.; Chen, F.; Callari, M.; Pourgholami, M.; Morris, D.L.; Stenzel, M.H. PEGylated Albumin based Polyion Complex Micelles for protein delivery. Biomacromolecules 2016, 17, 808–817. [Google Scholar] [CrossRef]
- Sun, Y.; Kiang, C. DNA-based Artificial Nanostructures: Fabrication, Properties and Apllications. In Handbook of Nanostructured Biomaterials and Their Applications in Nanobiotechnology; Chapter V; American Scientific Publishers: Nalwa, India, 2005. [Google Scholar]
- Xu, J.; Craig, S.L. Thermodynamics of DNA Hybridization on Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 13227–13231. [Google Scholar] [CrossRef] [PubMed]
- Furst, A.L.; Klass, S.H.; Francis, M.B. DNA Hybridization to Control Cellular Interactions. Trends Biochem. Sci. 2018, 44, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines. Theranostics 2020, 8, 4016–4032. [Google Scholar] [CrossRef]
- Sun, X.; Liu, M.; Chen, X.; Lin, H.; Liu, B.; Peng, Y.; Zhang, L.; Shen, J.; Zhao, P. A dual-targeted nucleic acid moiety decorated SPION nanoparticles for chemo-photodynamic synergistic therapy. J. Lumin. 2019, 209, 387–397. [Google Scholar] [CrossRef]





| Applications of NPs Drug Delivery Systems | Advantages |
|---|---|
| Tumors | Passive targeting promoted by enhanced permeability and retention (EPR) effect: accumulation on target site is favored |
| Diabetes | Less-invasive route for the delivery of insulin and improvement in the drug delivery |
| Cardiovascular Diseases | Development of regenerative medicine and of the possibility to manage this kind of disease |
| CNS Injuries | Possibility to overcome biological barriers, reaching the CNS and having a less-invasive delivery system |
| Coating Strategies | Advantages | Disadvantages |
|---|---|---|
| Polymers |
|
|
| Cell Membranes |
|
|
| Proteins and Antibodies |
|
|
| Hybridized DNA Structure |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinelli, F.; Perale, G.; Rossi, F. Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery. Gels 2020, 6, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6010006
Pinelli F, Perale G, Rossi F. Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery. Gels. 2020; 6(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6010006
Chicago/Turabian StylePinelli, Filippo, Giuseppe Perale, and Filippo Rossi. 2020. "Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery" Gels 6, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6010006