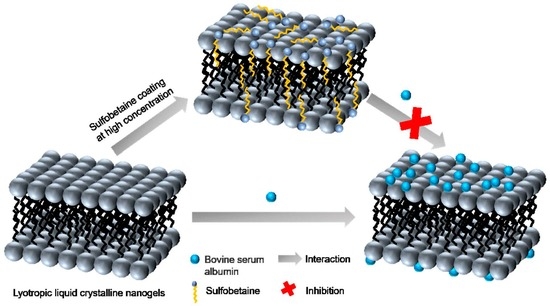
The Effect of Sulfobetaine Coating in Inhibiting the Interaction between Lyotropic Liquid Crystalline Nanogels and Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Size and ζ-Potential of GLLCNs and GLLCNs@HDSB
2.2. UV-Vis Spectra of GLLCNs and GLLCNs@HDSB after Incubation with BSA
2.3. Fluorescence Spectra of GLLCNs and GLLCNs@HDSB after Incubation with BSA
2.4. CD of GLLCNs and GLLCNs@HDSB following BSA Incubation
2.5. Analysis of the Interaction Modes
2.6. Inspiration for Future Application
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of LLCNs
4.3. The Interaction between GLLCNs and GLLCNs@HDSB with BSA
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhang, W. The Janus of Protein Corona on nanoparticles for tumor targeting, immunotherapy and diagnosis. J. Control. Release 2022, 345, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, C.; Yong, T.; Li, Z.; Gan, L.; Yang, X. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 2020, 49, 2273–2290. [Google Scholar] [CrossRef] [PubMed]
- De Maar, J.; Sofias, A.M.; Siegel, T.P.; Vreeken, R.J.; Moonen, C.; Bos, C.; Deckers, R. Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour environment. Theranostics 2020, 10, 1884–1909. [Google Scholar] [CrossRef] [PubMed]
- Adityan, S.; Tran, M.; Bhavsar, C.; Wu, S.Y. Nano-therapeutics for modulating the tumour microenvironment: Design, development, and clinical translation. J. Control. Release 2020, 327, 512–532. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, Z. New Strategies in the Design of Nanomedicines to Oppose Uptake by the Mononuclear Phagocyte System and Enhance Cancer Therapeutic Efficacy. Chem. Asian J. 2018, 13, 3333–3340. [Google Scholar] [CrossRef]
- Vu, T.N.; Le, P.H.P.; Pham, D.N.P.; Hoang, T.H.; Nadda, A.K.; Le, T.S.; Pham, T.D. Highly adsorptive protein inorganic nanohybrid of Moringa seeds protein and rice husk nanosilica for effective adsorption of pharmaceutical contaminants. Chemosphere 2022, 307 Pt 2, 135856. [Google Scholar] [CrossRef]
- Isaia, H.A.; Pinilla, C.M.B.; Brandelli, A. Evidence that protein corona reduces the release of antimicrobial peptides from polymeric nanocapsules in milk. Food Res. Int. 2020, 140, 110074. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, C.; Zhang, T. FcγRIIB receptor-mediated apoptosis in macrophages through interplay of cadmium sulfide nanomaterials and protein corona. Ecotoxicol. Environ. Saf. 2018, 164, 140–148. [Google Scholar] [CrossRef]
- Huang, Y.; Yamaguchi, A.; Pham, T.D.; Kobayashi, M. Charging and aggregation behavior of silica particles in the presence of lysozymes. Colloid Polym. Sci. 2017, 296, 145–155. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2011, 134, 2139–2147. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Kim, H.R.; Andrieux, K.; Delomenie, C.; Chacun, H.; Appel, M.; Desmaële, D.; Taran, F.; Georgin, D.; Couvreur, P.; Taverna, M. Analysis of plasma protein adsorption onto PEGylated nanoparticles by complementary methods: 2-DE, CE and Protein Lab-on-chip® system. Electrophoresis 2007, 28, 2252–2261. [Google Scholar] [CrossRef]
- Debayle, M.; Balloul, E.; Dembele, F.; Xu, X.; Hanafi, M.; Ribot, F.; Monzel, C.; Coppey, M.; Fragola, A.; Dahan, M.; et al. Zwitterionic polymer ligands: An ideal surface coating to totally suppress protein-nanoparticle corona formation? Biomaterials 2019, 219, 119357. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Parish, C.M.; Henry, J.; Katoh, Y. High throughput crystal structure and composition mapping of crystalline nanoprecipitates in alloys by transmission Kikuchi diffraction and analytical electron microscopy. Ultramicroscopy 2019, 202, 33–43. [Google Scholar] [CrossRef]
- Spicer, P.T. Progress in liquid crystalline dispersions: Cubosomes. Curr. Opin. Colloid Interface Sci. 2005, 10, 274–279. [Google Scholar] [CrossRef]
- Murgia, S.; Biffi, S.; Mezzenga, R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr. Opin. Colloid Interface Sci. 2020, 48, 28–39. [Google Scholar] [CrossRef]
- Waheed, A.; Aqil, M. Lyotropic liquid crystalline nanoparticles: Scaffolds for delivery of myriad therapeutics and diagnostics. J. Mol. Liq. 2021, 338, 116919. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Silva, C.H.T.P.; Imasato, H.; Tabak, M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: Spectroscopy and modelling. Biochim. Biophys. Acta 2002, 1594, 84–99. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Synthesis and characterization of manganese ferrite nanoparticles, and its interaction with bovine serum albumin: A spectroscopic and molecular docking approach. J. Mol. Liq. 2019, 296, 111871. [Google Scholar] [CrossRef]
- Chruszcz, M.; Mikolajczak, K.; Mank, N.; Majorek, K.A.; Porebski, P.J.; Minor, W. Serum albumins—Unusual allergens. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5375–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.; Huang, Z.; Wang, W.; Ma, X.; Wang, L.; Huang, Y.; Hu, P.; Pan, X.; Wu, C. Interaction between bovine serum albumin and Solutol® HS 15 micelles: A two-stage and concentration-dependent process. J. Drug Deliv. Sci. Technol. 2021, 64, 102376. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Jovanović, D.; Arsenijević, N.; Laban, B.; Pašti, T.L.; Klekotka, U.; Bondžić, B.P. “Soft Protein Corona” as the Stabilizer of the Methionine-Coated Silver Nanoparticles in the Physiological Environment: Insights into the Mechanism of the Interaction. Int. J. Mol. Sci. 2022, 23, 8985. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Z.; Huang, Z.; Fu, F.; Wu, L.; Huang, Y.; Wu, C.; Pan, X. Two Different Protein Corona Formation Modes on Soluplus® Nanomicelles. Colloids Surf. B Biointerfaces 2022, 218, 112744. [Google Scholar] [CrossRef]
- Woźniak-Budych, M.J.; Przysiecka, Ł.; Maciejewska, B.M.; Wieczorek, D.; Staszak, K.; Jarek, M.; Jesionowski, T.; Jurga, S. Facile Synthesis of Sulfobetaine-Stabilized Cu2O Nanoparticles and Their Biomedical Potential. ACS Biomater. Sci. Eng. 2017, 3, 3183–3194. [Google Scholar] [CrossRef]
- Roufik, S.; Gauthier, S.F.; Dufour, A.; Turgeon, S.L. Interactions between Bovine β-Lactoglobulin A and Various Bioactive Peptides As Studied by Front-Face Fluorescence Spectroscopy. J. Agric. Food Chem. 2006, 54, 4962–4969. [Google Scholar] [CrossRef]
- Ashraf, S.; Park, J.; Bichelberger, M.A.; Kantner, K.; Hartmann, R.; Maffre, P.; Said, A.H.; Feliu, N.; Lee, J.; Lee, D.; et al. Zwitterionic surface coating of quantum dots reduces protein adsorption and cellular uptake. Nanoscale 2016, 8, 17794–17800. [Google Scholar] [CrossRef]
- Ojha, H.; Mishra, K.; Hassan, M.I.; Chaudhury, N.K. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim. Acta 2012, 548, 56–64. [Google Scholar] [CrossRef]
- Estephan, Z.G.; Schlenoff, P.S.; Schlenoff, J.B. Zwitteration As an Alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [Google Scholar] [CrossRef]
- Alallam, B.; Doolaanea, A.A.; Oo, M.K.; Nasir, M.H.M.; Taher, M. Influence of nanoparticles surface coating on physicochemical properties for CRISPR gene delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102910. [Google Scholar] [CrossRef]
- Nikoo, A.M.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Electrospray-assisted encapsulation of caffeine in alginate microhydrogels. Int. J. Biol. Macromol. 2018, 116, 208–216. [Google Scholar] [CrossRef]
- Moreira, L.M.; Santiago, P.S.; de Almeida, V.; Tabak, M. Interaction of giant extracellular Glossoscolex paulistus hemoglobin (HbGp) with zwitterionic surfactant N-hexadecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (HPS): Effects of oligomeric dissociation. Colloids Surf. B Biointerfaces 2008, 61, 153–163. [Google Scholar] [CrossRef]
- He, X.; Li, Q.; Liu, X.; Wu, G.; Zhai, G. Curcumin-Loaded Lipid Cubic Liquid Crystalline Nanoparticles: Preparation, Optimization, Physicochemical Properties and Oral Absorption. J. Nanosci. Nanotechnol. 2015, 15, 5559–5565. [Google Scholar] [CrossRef]
- Maiorova, L.A.; Erokhina, S.I.; Pisani, M.; Barucca, G.; Marcaccio, M.; Koifman, O.I.; Salnikov, D.S.; Gromova, O.A.; Astolfi, P.; Ricci, V.; et al. Encapsulation of vitamin B12 into nanoengineered capsules and soft matter nanosystems for targeted delivery. Colloids Surf. B Biointerfaces 2019, 182, 110366. [Google Scholar] [CrossRef]





| Molecular Weight | Isoelectric Point | Grand Average of Hydropathicity | Amino Acid Residue |
|---|---|---|---|
| 69,222 | 5.82 | −0.433 | 583 |
| Sample Name | DH (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| GLLCNs | 135.30 ± 3.84 | 0.2047 ± 0.0121 | −16.00 ± 0.26 |
| GLLCNs@HDSB | 207.10 ± 2.14 | 0.2000 ± 0.0080 | −1.65 ± 0.05 |
| Spectrum | At Low Concentrations | At High Concentrations | Conclusion |
|---|---|---|---|
| UV-Vis | No significant difference | The increase in the GLLCNs@HDSB group was greater than that in the GLLCNs group | At low concentrations GLLCNs@HDSB couldn’t inhibit the interaction between nanogels and proteins, only at high concentrations GLLCNs@HDSB produces inhibitory interactions |
| Fluorescence | The fluorescence quenching degree of the GLLCNs@HDSB group was weaker than that of the GLLCNs group | The fluorescence quenching intensity of the GLLCNs@HDSB group was greater than that of the GLLCNs group | |
| CD | The conformational changes of the GLLCNs and GLLCNs@HDSB group were not significantly different | The ellipticity values in the GLLCNs@HDSB group were smaller than those in the GLLCNs group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Chen, Z.; Xie, Y.; Wang, W.; Huang, Z.; Huang, Y.; Wu, C.; Pan, X. The Effect of Sulfobetaine Coating in Inhibiting the Interaction between Lyotropic Liquid Crystalline Nanogels and Proteins. Gels 2022, 8, 653. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8100653
Zhong Z, Chen Z, Xie Y, Wang W, Huang Z, Huang Y, Wu C, Pan X. The Effect of Sulfobetaine Coating in Inhibiting the Interaction between Lyotropic Liquid Crystalline Nanogels and Proteins. Gels. 2022; 8(10):653. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8100653
Chicago/Turabian StyleZhong, Ziqiao, Zhiwei Chen, Yuke Xie, Wenhao Wang, Zhengwei Huang, Ying Huang, Chuanbin Wu, and Xin Pan. 2022. "The Effect of Sulfobetaine Coating in Inhibiting the Interaction between Lyotropic Liquid Crystalline Nanogels and Proteins" Gels 8, no. 10: 653. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8100653