Smart-Temporary-Film-Based Local-Delivery System with Controllable Drug-Release Behavior
Abstract
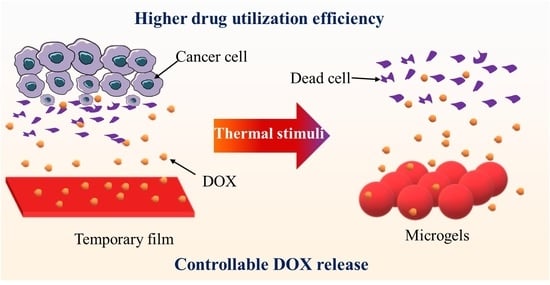
:1. Introduction
2. Results and Discussion
2.1. Morphologies
2.2. Thermal and Crystal Properties
2.3. Drug-Release Behavior
2.4. Cytotoxicity and Blood Compatibility
2.5. Antitumor Activities
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Temporary Film
4.3. Characterization
4.4. Drug-Release Behavior
4.5. Cytocompatibility Test
4.6. Assessment of Blood Compatibility
4.7. Antitumor Experiments
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for antitumor implantable drug delivery systems: Recent advances and future outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ma, Z.; Yuan, K.; Ji, T. Using scaffolds as drug delivery systems to treat bone tumor. Nanotechnology 2022, 33, 212002. [Google Scholar] [CrossRef]
- Hope, A.; Wade, S.J.; Aghmesheh, M.; Vine, K.L. Localized delivery of immunotherapy via implantable scaffolds for breast cancer treatment. J. Control Release 2022, 341, 399–413. [Google Scholar] [CrossRef]
- Brudno, Y.; Mooney, D.J. On-demand drug delivery from local depots. J. Control Release 2015, 219, 8–17. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. NRM 2016, 2, 16075. [Google Scholar] [CrossRef]
- Qu, Y.; Chu, B.Y.; Peng, J.R.; Liao, J.F.; Qi, T.T.; Shi, K.; Zhang, X.N.; Wei, Y.Q.; Qian, Z.Y. A biodegradable thermo-responsive hybrid hydrogel: Therapeutic applications in preventing the post-operative recurrence of breast cancer. NPG Asia Mater. 2015, 7, e207. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhang, Z.; Zhang, Y.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Use of asymmetric multilayer polylactide nanofiber mats in controlled release of drugs and prevention of liver cancer recurrence after surgery in mice. Nanomedicine 2015, 11, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, X.; Yin, J.; Mou, Y.; Huang, H.; Ren, Z. Selective delivery of curcumin to breast cancer cells by self-targeting apoferritin nanocages with pH-responsive and low toxicity. Drug Deliv. 2022, 29, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zhang, X.; Yu, S.; Wen, D.; Hu, Q.; Ye, Y.; Bomba, H.; Hu, X.; Liu, Z. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018, 10, eaan3682. [Google Scholar] [CrossRef] [Green Version]
- Kubota, T.; Kurashina, Y.; Zhao, J.; Ando, K.; Onoe, H. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer. Mater. Design 2021, 203, 109580. [Google Scholar] [CrossRef]
- Bu, L.L.; Yan, J.; Wang, Z.; Ruan, H.; Chen, Q.; Gunadhi, V.; Bell, R.B.; Gu, Z. Advances in drug delivery for post-surgical cancer treatment. Biomaterials 2019, 219, 119182. [Google Scholar] [CrossRef]
- Lendlein, A.; Gould, O.E. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Yuan, J.; Neri, W.; Zakri, C.; Merzeau, P.; Kratz, K.; Lendlein, A.; Poulin, P. Shape memory nanocomposite fibers for untethered high-energy microengines. Science 2019, 365, 155–158. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Ao, H.; Luo, H.; Deng, X.; Wan, Y. Embedding carbon nanotube to the surfaces of poly(ε-caprolactone) film for multi-responsive actuations. Polym. Test. 2021, 96, 107086. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Retentive drug delivery systems based on shape memory materials. J. Appl. Polym. Sci. 2020, 137, 48798. [Google Scholar] [CrossRef]
- Liu, Y.; Gould, O.E.C.; Kratz, K.; Lendlein, A. On demand sequential release of (sub)micron particles controlled by size and temperature. Small 2022, 18, e2104621. [Google Scholar] [CrossRef]
- Zhang, Q.; Sauter, T.; Fang, L.; Kratz, K.; Lendlein, A. Shape-memory capability of copolyetheresterurethane microparticles prepared via electrospraying. Macromol. Mater. Eng. 2015, 300, 522–530. [Google Scholar] [CrossRef]
- Zhang, Q.; Kratz, K.; Lendlein, A. Shape-memory properties of degradable electrospun scaffolds based on hollow microfibers. Polym. Adv. Technol. 2015, 26, 1468–1475. [Google Scholar] [CrossRef]
- Zhang, Q.; Rudolph, T.; Benitez, A.J.; Gould, O.E.C.; Behl, M.; Kratz, K.; Lendlein, A. Temperature-controlled reversible pore size change of electrospun fibrous shape-memory polymer actuator based meshes. Smart Mater. Struct. 2019, 28, 055037. [Google Scholar] [CrossRef]
- Nonkrathok, W.; Trongsatitkul, T.; Suppakarn, N. Role of maleic anhydride-grafted poly(lactic acid) in improving shape memory properties of thermoresponsive poly(ethylene glycol) and poly(lactic acid) blends. Polymers 2022, 14, 3923. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.C.; Zhang, J.J.; Zhou, Y.; Song, B.Y.; Chang, J.J.; Yang, K.K.; Wang, Y.Z. Biodegradable poly(p-dioxanone) reinforced and toughened by organo-modified vermiculite. Polym. Adv. Technol. 2011, 22, 993–1000. [Google Scholar] [CrossRef]
- Ren, T.; Feng, C.; Dong, J.; Zhu, H.; Wang, B. Preparation and in vivo bacteriostatic application of PPDO-coated Ag loading TiO2 nanoparticles. Sci. Rep. 2022, 12, 10585. [Google Scholar] [CrossRef]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; McFarlane, J.D. Extracellular pH distribution in human tumours. Int. J. Hyperther. 1995, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, Y.; Chen, G.; Wang, H.; Shao, C.; Chen, Z.; Lu, P.; Zhao, Y. Mesoporous colloidal photonic crystal particles for intelligent drug delivery. ACS Appl. Mater. Inter. 2018, 10, 33936–33944. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Yang, Z.; Zuo, G.; Zhang, Q.; Yao, F.; Wan, Y. Encapsulating doxorubicin-intercalated lamellar nanohydroxyapatite into PLGA nanofibers for sustained drug release. Curr. Appl. Phys. 2019, 19, 1204–1210. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, Y.; Gan, D.; Zhang, Q.; Luo, H.; Deng, X.; Li, Z.; Yang, Z. Enwrapping polydopamine on doxorubicin-loaded lamellar hydroxyapatite/poly(lactic-co-glycolic acid) composite fibers for inhibiting bone tumor recurrence and enhancing bone regeneration. ACS Appl. Bio. Mater. 2021, 4, 6036–6045. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, S.; Shen, M.; Zhu, M.; Shi, X. Antitumor efficacy of doxorubicin-loaded electrospun nano-hydroxyapatite–poly(lactic-co-glycolic acid) composite nanofibers. Polym. Chem. 2013, 4, 933–941. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Zamani, M.; Felice, B.; Ramakrishna, S. Electrospraying technique for the fabrication of metronidazole contained PLGA particles and their release profile. Mater. Sci. Eng. C 2015, 56, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Elmay, W.; Patoor, E.; Gloriant, T.; Prima, F.; Laheurte, P. Improvement of superelastic performance of Ti-Nb binary alloys for biomedical applications. J. Mater. Eng. Perform. 2014, 23, 2471–2476. [Google Scholar] [CrossRef]
- Das, G.; Nicastri, A.; Coluccio, M.L.; Gentile, F.; Candeloro, P.; Cojoc, G.; Liberale, C.; De Angelis, F.; Di Fabrizio, E. FT-IR, Raman, RRS measurements and DFT calculation for doxorubicin. Microsc. Res. Techniq. 2010, 73, 991–995. [Google Scholar] [CrossRef]
- Wang, D.; Xu, X.; Zhang, K.; Sun, B.; Wang, L.; Meng, L.; Liu, Q.; Zheng, C.; Yang, B.; Sun, H. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int. J. Nanomed. 2018, 13, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, Q.; Dai, L.; Shen, X.; Chen, W.; Cai, K. Phenylboronic acid-modified hollow silica nanoparticles for dual-responsive delivery of doxorubicin for targeted tumor therapy. Regen. Biomater. 2017, 4, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-Y.; Ju, T.-K.; Chen, N.G.; Hsiao, C.-J.; Wang, C.-Y. Ultrasound-enhanced delivery of an aptamer-doxorubicin conjugate to breast cancer cells. IEEE Access 2019, 7, 94189–94194. [Google Scholar] [CrossRef]
- Grablowitz, H.; Lendlein, A. Synthesis and characterization of α,ω-dihydroxy-telechelic oligo(p-dioxanone). J. Mater. Chem. 2007, 17, 4050–4056. [Google Scholar] [CrossRef]
- Ming, Z.; Ma, S.; Xu, K.; Chu, P.K. Corrosion resistance of praseodymium-ion-implanted TiN coatings in blood and cytocompatibility with vascular endothelial cells. Vacuum 2015, 117, 73–80. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, D.; Wang, H.; Yin, C.; Peng, M.; Ao, H.; Hu, J.; Wan, Y.; Zhang, Q. Smart-Temporary-Film-Based Local-Delivery System with Controllable Drug-Release Behavior. Gels 2022, 8, 773. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8120773
Xie D, Wang H, Yin C, Peng M, Ao H, Hu J, Wan Y, Zhang Q. Smart-Temporary-Film-Based Local-Delivery System with Controllable Drug-Release Behavior. Gels. 2022; 8(12):773. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8120773
Chicago/Turabian StyleXie, Denghang, Huiwen Wang, Cheng Yin, Mengxia Peng, Haiyong Ao, Jian Hu, Yizao Wan, and Quanchao Zhang. 2022. "Smart-Temporary-Film-Based Local-Delivery System with Controllable Drug-Release Behavior" Gels 8, no. 12: 773. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8120773