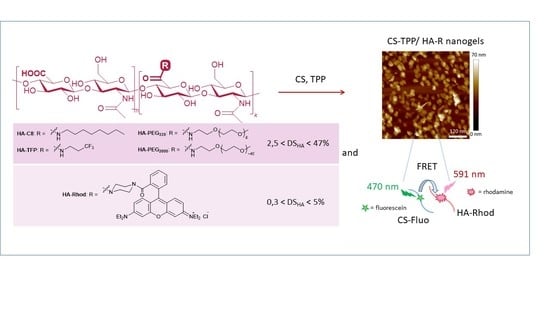
Synthesis and Characterization of Conjugated Hyaluronic Acids. Application to Stability Studies of Chitosan-Hyaluronic Acid Nanogels Based on Fluorescence Resonance Energy Transfer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Functionalization of Hyaluronic Acid
2.1.1. Grafting of n-Octylamine on Hyaluronic Acid
2.1.2. Grafting of Polyethyleneglycol Oligomers on Hyaluronic Acid
2.1.3. Grafting of Trifluoropropylamine on Hyaluronic Acid
2.1.4. Grafting of Rhodamine B Amine on Hyaluronic Acid
2.2. Nanogel Syntheses with Functionalized HA and Characterization—Evaluation of Nanogel Stability by Förster Energy Transfer Experiments (FRET)
2.2.1. Nanogel Synthesis with Functionalized HA and Characterization
2.2.2. Evaluation of Nanogels Stability by FRET Experiments
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Syntheses and Purifications of Functionalized HA
4.2.1. Conversion of Sodium Hyaluronate HAs into Its Protonated Form HAp
4.2.2. General Method of HA Functionalization by Peptidic Coupling Reaction with Amine Synthons in DMSO
4.3. Determination of Functionalized HA Degree of Substitution DSHA
4.4. Preparation of Nanoparticles with Functionalized HA Polymers by Ionic Gelation and Characterizations
4.4.1. CS-TPP/Functionalized HA Nanogel Synthesis
4.4.2. Nanogels Characterization by Dynamic Light Scattering (DLS)
4.4.3. Atomic Force Microscopy
4.4.4. Evaluation of CS-Fluo-TPP/HA-Rhod Nanogels Stability by Förster Resonance Energy Transfer (FRET) Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliver. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiel, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabrala, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Khan, W.; Abtew, E.; Modani, S.; Domb, A.J. Polysaccharide based nanoparticles. Isr. J. Chem. 2018, 58, 1315–1329. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Shufen, Z.; Zhang, Z. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef]
- Wolf, K.J.; Kumar, S. Hyaluronic Acid: Incorporating the Bio into the Material. ACS Biomater. Sci. Eng. 2019, 5, 3753–3765. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Kirschning, A.; Dibbert, N.; Drager, G. Chemical functionalization of polysaccharides—Towards biocompatible hydrogels for biomedical applications. Chem. Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef]
- Rho, J.G.; Han, H.S.; Han, J.H.; Lee, H.; Nguyen, V.Q.; Lee, W.H.; Kim, W. Self-assembled hyaluronic acid nanoparticles: Implications as a nanomedicine for treatment of type 2 diabetes. J. Control. Release 2018, 279, 89–98. [Google Scholar] [CrossRef]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.-H.; Ryu, J.-H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, V.D.; Maheriya, P.M. Hyaluronic acid as potential carrier in biomedical and drug delivery applications. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–265. [Google Scholar]
- Kaewruethai, T.; Laomeephol, C.; Pan, Y.; Luckanagul, J.A. Multifunctional Polymeric Nanogels for Biomedical Applications. Gels 2021, 7, 228. [Google Scholar] [CrossRef]
- Yuan, J.; Maturavongsadit, P.; Zhou, Z.; Lv, B.; Lin, Y.; Yang, J.; Luckanagul, J. Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats. Biomater Transl. 2020, 1, 89–98. [Google Scholar]
- Jia, X.; Han, Y.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. Multi-functionalized hyaluronic acid nanogels crosslinked with carbon dots as dual receptor-mediated targeting tumor theranostics. Carbohydr. Polym. 2016, 152, 391–397. [Google Scholar] [CrossRef]
- Silva Garcia, J.M.; Panitch, A.; Calve, S. Functionalization of hyaluronic acid hydrogels with ECM-derived peptides to control myoblast behavior. Acta Biomater. 2019, 84, 169–179. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Wang, Y.; Gu, X.; Wang, G.; Li, J. Hyaluronic acid-functionalized half-generation of sectorial dendrimers for anticancer drug delivery and enhanced biocompatibility. Carbohydr. Polym. 2018, 202, 513–522. [Google Scholar] [CrossRef]
- Crescenzi, V.; Francescangeli, A.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. Hyaluronan networking via Ugi’s condensation using lysine as cross-linker diamine. Carbohydr. Polym. 2003, 53, 311–316. [Google Scholar] [CrossRef]
- Ramachandran, B.; Chakraborty, S.; Kannan, R.; Dixit, M.; Muthuvijayan, V. Immobilization of hyaluronic acid from Lactococcus lactis on polyethylene terephthalate for improved biocompatibility and drug release. Carbohydr. Polym. 2019, 206, 132–140. [Google Scholar] [CrossRef]
- Vasi, A.-M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef]
- Wei, K.; Zhu, M.; Sun, Y.; Xu, J.; Feng, Q.; Lin, S.; Wu, T.; Xu, J.; Tian, F.; Xia, J.; et al. Robust Biopolymeric Supramolecular “Host−Guest Macromer” Hydrogels Reinforced by in Situ Formed Multivalent Nanoclusters for Cartilage Regeneration. Macromolecules 2016, 49, 866–875. [Google Scholar] [CrossRef]
- Liu, C.; Liu, D.; Wang, Y.; Li, Y.; Li, T.; Zhou, Z.; Yang, Z.; Wang, J.; Zhang, Q. Glycol chitosan/oxidized hyaluronic acid hydrogels functionalized with cartilage extracellular matrix particles and incorporating BMSCs for cartilage repair. Artif. Cells Nanomed. Biotechnol. 2018, 46, 721–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Q.; Fan, Y.; Hu, Y.; Cheng, G.; Xu, F.-J. Polysaccharide–Peptide Conjugates: A Versatile Material Platform for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005978. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconjugate Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef]
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs. EDC/NHS for ligation of amines to hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246. [Google Scholar] [CrossRef]
- Yan, Q.; Zheng, H.-N.; Jiang, C.; Li, K.; Xiao, S.-J. EDC/NHS activation mechanism of polymethacrylic acid: Anhydride versus NHS-ester. RSC Adv. 2015, 5, 69939–69947. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef]
- Almeida, P.V.; Shahbazi, M.-A.; Mäkilä, E.; Kaasalainen, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. Amine-modified hyaluronic acid-functionalized porous silicon nanoparticles for targeting breast cancer tumors. Nanoscale 2014, 6, 10377–10387. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Picart, C.; Senger, B.; Schaaf, P.; Voegel, J.; Frisch, B. Layer-by-Layer Films from Hyaluronan and Amine-Modified Hyaluronan. Langmuir 2007, 23, 2655–2662. [Google Scholar] [CrossRef] [Green Version]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Hydrogels Incorporating GdDOTA: Towards Highly Efficient Dual T1/T2 MRI Contrast Agents. Angew. Chem. Int. Ed. 2012, 51, 9119–9122. [Google Scholar] [CrossRef]
- Callewaert, M.; Roullin, V.G.; Cadiou, C.; Millart, E.; Van Gulik, L.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; Laurent, L.; Vander Elst, L.; et al. Tuning the composition of biocompatible Gd nanohydrogels to achieve hypersensitive dual T1/T2 MRI contrast agents. J. Mater. Chem. B 2014, 2, 6397–6405. [Google Scholar] [CrossRef]
- Malytskyi, V.; Moreau, J.; Callewaert, M.; Rigaux, G.; Cadiou, C.; Laurent, S.; Chuburu, F. Organic nanoparticles and gadolinium chelates. In Materials for Biomedical Engineering: Organic Micro and Nanostructures, 1st ed.; Grumezescu, A., Holban, A.-M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–476. [Google Scholar]
- Moreau, J.; Callewaert, M.; Malytskyi, V.; Henoumont, C.; Voicu, S.N.; Stan, M.S.; Molinari, M.; Cadiou, C.; Laurent, S.; Chuburu, F. Fluorescent chitosan-based nanohydrogels and encapsulation of gadolinium MRI contrast agent for magneto-optical imaging. Carbohydr. Polym. Technol. Appl. 2021, 2, 100104. [Google Scholar]
- Chib, R.; Raut, S.; Fudala, R.; Chang, A.; Mummert, M.; Rich, R.; Gryczynski, Z.; Gryczynski, I. FRET Based-Metric Sensing of Hyaluronidase in Synthetic Urine as a Biomarker for Bladder and Prostate Cancer. Curr. Pharm. Biotechnol. 2013, 14, 470–474. [Google Scholar] [CrossRef] [Green Version]
- Fudala, R.; Mummert, M.E.; Gryczynski, Z.; Gryczynski, I. Fluorescence detection of hyaluronidase. J. Photochem. Photobiol. B 2011, 104, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Belabassi, Y.; Moreau, J.; Gheran, V.; Henoumont, C.; Robert, A.; Callewaert, M.; Rigaux, G.; Cadiou, C.; Vander Elst, L.; Laurent, S.; et al. Synthesis and characterization of PEGylated and fluorinated chitosans: Application to the synthesis of targeted nanoparticles for drug delivery. Biomacromolecules 2017, 18, 2756–2766. [Google Scholar] [CrossRef]
- Eslami, P.; Rossi, F.; Fedeli, S. Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics 2019, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, L.; Alvarez-Puebla, R.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Misselwitz, B. MR contrast agents in lymph node imaging. Eur. J. Radiol. 2006, 35, 375–382. [Google Scholar] [CrossRef]
- Kačenka, M.; Kaman, O.; Kikerlová, S.; Pavlů, B.; Jirák, Z.; Jirák, D.; Herynek, V.; Černý, J.; Chaput, F.; Laurent, S.; et al. Fluorescent magnetic nanoparticles for cell labeling: Flux synthesis of manganite particles and novel functionalization of silica shell. J. Colloid Interface Sci. 2015, 447, 97–106. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan–hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef]
- Rigaux, G.; Gheran, C.V.; Callewaert, M.; Cadiou, C.; Voicu, S.N.; Dinischiotu, A.; Andry, M.C.; Vander Elst, L.; Laurent, S.; Muller, R.N.; et al. Characterization of Gd loaded chitosan-TPP nanohydrogels by a multi-technique approach combining dynamic light scattering (DLS), asymetrical flow-field-flow fractionation (AF4) and atomic force microscopy (AFM) and design of positive contrast agents for molecular resonance imaging (MRI). Nanotechnology 2017, 28, 055705. [Google Scholar]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomic, structures and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, M.D.; Merzouki, A.; Lavertu, M.; Thibault, M.; Jean, M.; Darras, V. Chitosans for delivery of nucleic acids. Adv. Drug Deliv. Rev. 2013, 65, 1234–1270. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Vårum, K.M.; Antohonsen, M.W.; Grasdalen, H.; Smidsrød, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field N.M.R. spectroscopy. Carbohydr. Res. 1991, 211, 17–23. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Pitarresi, G.; Mandracchia, D.; Tripodo, G.; Giammona, G. New graft copolymers of hyaluronic acid and polylactic acid: Synthesis and characterization. Carbohydr. Polym. 2006, 66, 379–385. [Google Scholar] [CrossRef]
- Cho, H.-J.; Yoon, H.Y.; Koo, H.; Ko, S.-H.; Shim, J.-S.; Cho, J.-H.; Park, J.H.; Kim, K.; Kwon, I.C.; Kim, D.-D. Hyaluronic acid-ceramide-based optical/MR dual imaging nanoprobe for cancer diagnosis. J. Control. Release 2012, 162, 111–118. [Google Scholar] [CrossRef]
- Johnson, C.S., Jr. Diffusion Ordered Nuclear Magnetic Resonance Spectroscopy: Principles and Applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Augé, S.; Amblard-Blondel, B.; Delsuc, M.A. Investigation of the diffusion measurement using PFG and tTest r against experimental conditions and parameters. J. Chim. Phys. Phys.-Chim. Biol. 1999, 96, 1559–1565. [Google Scholar] [CrossRef]
- Best, J.P.; Neubauer, M.P.; Javed, S.; Dam, H.H.; Fery, A.; Caruso, F. Mechanics of pH-Responsive Hydrogel Capsules. Langmuir 2013, 29, 9814–9823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Björnmalm, M.; Liang, K.; Xu, C.; Best, J.P.; Zhang, X.; Caruso, F. Super-Soft Hydrogel Particles with Tunable Elasticity in a Microfluidic Blood Capillary Model. Adv. Mater. 2014, 26, 7295–7299. [Google Scholar] [CrossRef] [PubMed]






| HA-C8a | HA-C8b | HA-C8c | HA-C8d | |
|---|---|---|---|---|
| % mol (amine/COOH)initial | 10% | 20% | 50% | 100% |
| Association rate (AR) 1 | 4.0% | 8.4% | 23.4% | 47.0% |
| Degree of substitution (DSHA) 2 | 1.9% | 7.2% | 22.4% | 42.9% |
| Grafting degree (GD) 3 | 71% | 92% | 100% | 100% |
| Degree of substitution (DSHA) 4 | 2.8% | 7.7% | 23.4% | 47.0% |
| HA-PEG339 | HA-PEG2000 | |||||||
|---|---|---|---|---|---|---|---|---|
| Entries | a | b | c | d | a | b | c | d |
| % mol (amine/COOH)initial | 10% | 20% | 50% | 100% | 10% | 20% | 50% | 100% |
| Degree of substitution (DSHA) 1 | 2.4% | 7.6% | 15.7% | 38.9% | 1.3% | 7.0% | 14.1% | 32.0% |
| HA-TFPa | HA-TFPb | HA-TFPc | HA-TFPd | |
|---|---|---|---|---|
| % mol (amine/COOH)initial | 10% | 20% | 50% | 100% |
| Degree of substitution (DSHA) a | 5.2% | 6.5% | 15.6% | 29.2% |
| HA-Rhod a | HA-Rhod b | HA-Rhod c | HA-Rhod d | |
|---|---|---|---|---|
| % mol (amine/COOH)initial | 10% | 20% | 50% | 100% |
| Association rate (AR) 1 | 1.1% | 1.9% | 4.0% | 7.6% |
| Grafting degree (GD) 2 | 25% | 62% | 71% | 64% |
| Degree of substitution (DSHA) 3 | 0.3% | 1.2% | 2.8% | 4.9% |
| Synthon | DSHA [%] | Z-Average ± sd (nm) | PdI ± sd | ζ ± sd (mV) |
|---|---|---|---|---|
| PEG339 | 2.4 | 132 ± 2 | 0.19 ± 0.01 | +26 ± 3 |
| 7.6 | 128 ± 1 | 0.18 ± 0.02 | +23 ± 4 | |
| 15.7 | 138 ± 2 | 0.19 ± 0.01 | +24 ± 3 | |
| 38.9 | 128 ± 1 | 0.17 ± 0.01 | +29 ± 3 | |
| PEG2000 | 1.3 | 141 ± 2 | 0.18 ± 0.02 | +26 ± 4 |
| 7 | 149 ± 1 | 0.18 ± 0.01 | +21 ± 3 | |
| 14.1 | 137 ± 2 | 0.19 ± 0.02 | +24 ± 4 | |
| 32 | 146 ± 1 | 0.18 ± 0.01 | +24 ± 3 | |
| TFB | 5.2 | 153 ± 3 | 0.18 ± 0.02 | +28 ± 3 |
| 6.5 | 140 ± 2 | 0.20 ± 0.01 | +25 ± 4 | |
| 15.6 | 148 ± 3 | 0.18 ± 0.02 | +23 ± 3 | |
| 29.2 | 146 ± 3 | 0.20 ± 0.01 | +23 ± 3 | |
| Rhod | 0.3 | 137 ± 3 | 0.18 ± 0.01 | +22 ± 3 |
| 1.2 | 147 ± 2 | 0.18 ± 0.02 | +23 ± 4 | |
| 2.8 | 148 ± 3 | 0.20 ± 0.01 | +23 ± 3 | |
| 4.9 | 141 ± 3 | 0.18 ± 0.02 | +21 ± 4 | |
| No synthon | 0 | 139 ± 2 | 0.18 ± 0.01 | +22 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malytskyi, V.; Moreau, J.; Callewaert, M.; Henoumont, C.; Cadiou, C.; Feuillie, C.; Laurent, S.; Molinari, M.; Chuburu, F. Synthesis and Characterization of Conjugated Hyaluronic Acids. Application to Stability Studies of Chitosan-Hyaluronic Acid Nanogels Based on Fluorescence Resonance Energy Transfer. Gels 2022, 8, 182. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8030182
Malytskyi V, Moreau J, Callewaert M, Henoumont C, Cadiou C, Feuillie C, Laurent S, Molinari M, Chuburu F. Synthesis and Characterization of Conjugated Hyaluronic Acids. Application to Stability Studies of Chitosan-Hyaluronic Acid Nanogels Based on Fluorescence Resonance Energy Transfer. Gels. 2022; 8(3):182. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8030182
Chicago/Turabian StyleMalytskyi, Volodymyr, Juliette Moreau, Maité Callewaert, Céline Henoumont, Cyril Cadiou, Cécile Feuillie, Sophie Laurent, Michael Molinari, and Françoise Chuburu. 2022. "Synthesis and Characterization of Conjugated Hyaluronic Acids. Application to Stability Studies of Chitosan-Hyaluronic Acid Nanogels Based on Fluorescence Resonance Energy Transfer" Gels 8, no. 3: 182. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8030182