Highly Responsive Chitosan-Co-Poly (MAA) Nanomatrices through Cross-Linking Polymerization for Solubility Improvement
Abstract
:1. Introduction
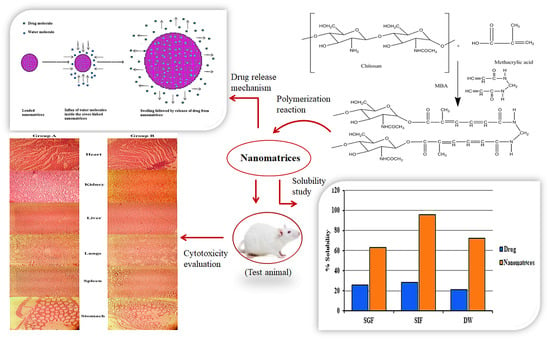
2. Results and Discussion
2.1. Sol-Gel Analysis and Stability Studies
2.2. Swelling Studies
2.3. Particle Size Analysis
2.4. SEM Analysis
2.5. Thermal Analysis of Nanomatrices
2.6. Powder X-ray Diffraction
2.7. Solubilization Efficacy
2.8. In Vitro Dissolution Studies
2.9. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.10. Acute Oral Toxicity Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Nanomatrices
4.2.2. Drug Loading of Nanomatrices
4.3. Characterization
4.3.1. Entrapment Efficiency
4.3.2. Solubility Study
4.3.3. Swelling Study
4.3.4. Sol-Gel Analysis
4.3.5. Particle Size Analysis
4.3.6. Stability Studies
4.3.7. Scanning Electron Microscopy
4.3.8. Fourier Transform Infrared Spectroscopy
4.3.9. Thermal Analysis
4.3.10. Powder X-ray Diffraction Analysis
4.3.11. In Vitro Drug Release Studies
4.4. Acute Oral Toxicity Studies
4.4.1. Animal Selection, Housing, and Preparation
4.4.2. Preparation and Administration of Dose
4.4.3. Clinical Observation
4.4.4. Hematology and Biochemical Blood Analysis
4.4.5. Histopathology Macroscopic Observation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chivere, V.T.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Nanotechnology-based biopolymeric oral delivery platforms for advanced cancer treatment. Cancers 2020, 12, 522. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, P.; Patravale, V. Dissolution enhancement of atorvastatin calcium by co-grinding technique. Drug Deliv. Transl. Res. 2016, 6, 380–391. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; Jain, D.K.; Saraf, A. Enhancement of oral bioavailability of poorly water soluble carvedilol by chitosan nanoparticles: Optimization and pharmacokinetic study. Int. J. Biol. Macromol. 2019, 135, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Q.; Ahmad, M.; Minhas, M.U.; Batool, F.; Malik, N.S.; Rehman, M. Novel β-cyclodextrin nanosponges by chain growth condensation for solubility enhancement of dexibuprofen: Characterization and acute oral toxicity studies. J. Drug Deliv. Sci. Technol. 2020, 61, 102089. [Google Scholar] [CrossRef]
- Jermain, S.V.; Brough, C.; Williamsn, R.O., III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef]
- Ndlovu, S.T.; Ullah, N.; Khan, S.; Ramharack, P.; Soliman, M.; De Matas, M.; Shahid, M.; Sohail, M.; Imran, M.; Shah, S.W.A.; et al. Domperidone nanocrystals with boosted oral bioavailability: Fabrication, evaluation and molecular insight into the polymer-domperidone nanocrystal interaction. Drug Deliv. Transl. Res. 2019, 9, 284–297. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-Influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Malviya, R.; Raj, S.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.; Kumari, U.; Meenakshi, D.U.; Porwal, O.; Kumar, D.H.; Singh, A.; et al. Evaluation of Antitumor Efficacy of Chitosan-Tamarind Gum Polysaccharide Polyelectrolyte Complex Stabilized Nanoparticles of Simvastatin. Int. J. Nanomed. 2021, 16, 2533–2553. [Google Scholar] [CrossRef] [PubMed]
- Knapik-Kowalczuk, J.; Chmiel, K.; Jurkiewicz, K.; Correia, N.T.; Sawicki, W.; Paluch, M. Physical Stability and Viscoelastic Properties of Co-Amorphous Ezetimibe/Simvastatin System. Pharmaceuticals 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, R.D.; Tieleman, D.P. Modulation of Phospholipid Bilayer Properties by Simvastatin. J. Phys. Chem. B 2021, 125, 8406–8418. [Google Scholar] [CrossRef]
- Ahmadi, M.; Amiribc, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; Da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic effects of statins: A focus on cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef]
- Duarte, J.A.; De Barros, A.L.B.; Leite, E.A. The potential use of simvastatin for cancer treatment: A review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef]
- Rohilla, A.; Rohilla, S.; Kumar, A.; Khan, M.; Deep, A. Pleiotropic effects of statins: A boulevard to cardioprotection. Arab. J. Chem. 2016, 9, S21–S27. [Google Scholar] [CrossRef] [Green Version]
- Bedi, O.; Dhawan, V.; Sharma, P.L.; Kumar, P. Pleiotropic effects of statins: New therapeutic targets in drug design. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2016, 389, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, H.; Pooja, D.; Prajapati, S.; Chauhan, A. Performance evaluation of PAMAM dendrimer based simvastatin formulations. Int. J. Pharm. 2011, 405, 203–209. [Google Scholar] [CrossRef]
- Anwar, M.; Warsi, M.H.; Mallick, N.; Akhter, S.; Gahoi, S.; Jain, G.K.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Enhanced bioavailability of nano-sized chitosan–atorvastatin conjugate after oral administration to rats. Eur. J. Pharm. Sci. 2011, 44, 241–249. [Google Scholar] [CrossRef]
- Rizvi, S.Z.H.; Shah, F.A.; Khan, N.; Muhammad, I.; Ali, K.H.; Ansari, M.M.; Din, F.U.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; et al. Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int. J. Pharm. 2019, 560, 136–143. [Google Scholar] [CrossRef]
- Pandya, P.; Gattani, S.; Jain, P.; Khirwal, L.; Surana, S. Co-solvent Evaporation Method for Enhancement of Solubility and Dissolution Rate of Poorly Aqueous Soluble Drug Simvastatin: In vitro–In vivo Evaluation. AAPS PharmSciTech 2008, 9, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Tres, F.; Hall, S.D.; Mohutsky, M.A.; Taylor, L.S. Monitoring the Phase Behavior of Supersaturated Solutions of Poorly Water-Soluble Drugs Using Fluorescence Techniques. J. Pharm. Sci. 2018, 107, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yu, X.; Yin, H. Study of Top-down and Bottom-up Approaches by Using Design of Experiment (DoE) to Produce Meloxicam Nanocrystal Capsules. AAPS PharmSciTech 2020, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Khattab, W.M.; El-Dein, E.E.Z.; El-Gizawy, S.A. Formulation of lyophilized oily-core poly-Ɛ-caprolactone nanocapsules to improve oral bioavailability of Olmesartan Medoxomil. Drug Dev. Ind. Pharm. 2020, 46, 795–805. [Google Scholar] [CrossRef]
- Kasekar, N.M.; Singh, S.; Jadhav, K.R.; Kadam, V.J. BCS Class II drug loaded protein nanoparticles with enhanced oral bioavailability: In vitro evaluation and in vivo pharmacokinetic study in rats. Drug Dev. Ind. Pharm. 2020, 46, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lim, L.M.; Dong, B.; Hadinoto, K. Proof-of-concept preparation and characterization of dual-drug amorphous nanoparticle complex as fixed-dose combination of poorly soluble drugs. Drug Dev. Ind. Pharm. 2019, 45, 105–116. [Google Scholar] [CrossRef]
- Badshah, S.F.; Akhtar, N.; Minhas, M.U.; Khan, K.U.; Khan, S.; Abdullah, O.; Naeem, A. Porous and highly responsive cross-linked β-cyclodextrin based nanomatrices for improvement in drug dissolution and absorption. Life Sci. 2021, 267, 118931. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Del Grosso, V.; Ferrari, G. Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels 2022, 8, 152. [Google Scholar] [CrossRef]
- Shahi, S.; Roghani-Mamaqani, H.; Talebi, S.; Mardani, H. Stimuli-responsive destructible polymeric hydrogels based on irreversible covalent bond dissociation. Polym. Chem. 2022, 13, 161–192. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A 2022, 110, 708–724. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef]
- Zia, M.A.; Sohail, M.; Minhas, M.U.; Sarfraz, R.M.; Khan, S.; de Matas, M.; Hussain, Z.; Abbasi, M.; Shah, S.A.; Kousar, M.; et al. HEMA based pH-sensitive semi IPN microgels for oral delivery; a rationale approach for ketoprofen. Drug Dev. Ind. Pharm. 2020, 46, 272–282. [Google Scholar] [CrossRef]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, S.; Akhtar, N.; Minhas, M.U.; Khan, K.U. Bi-polymeric Spongy Matrices Through Cross-linking Polymerization: Synthesized and Evaluated for Solubility Enhancement of Acyclovir. AAPS PharmSciTech 2021, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.S.B.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U. Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels 2022, 8, 55. [Google Scholar] [CrossRef]
- Khan, K.U.; Akhtar, N.; Minhas, M.U. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS PharmSciTech 2020, 21, 141. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Mutalik, S.; Anju, P.; Manoj, K.; Usha, A.N. Enhancement of dissolution rate and bioavailability of aceclofenac: A chitosan-based solvent change approach. Int. J. Pharm. 2008, 350, 279–2900. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Geçer, A.; Yıldız, N.; Çalımlı, A.; Turan, B. Trimethyl chitosan nanoparticles enhances dissolution of the poorly water soluble drug Candesartan-Cilexetil. Macromol. Res. 2010, 18, 986–991. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Mattoso, L.H. Preparation of chitosan nanoparticles using methacrylic acid. J. Colloid Interface Sci. 2008, 321, 477–483. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Synthesis and Characterization of Thermo- and Chemomechanically Responsive Poly(N-isopropylacrylamide-co-methacrylic acid) Hydrogels. Macromolecules 1995, 28, 8016–8020. [Google Scholar] [CrossRef]
- Pimpan, V.; Thothong, P. Synthesis of cassava starch-g-poly(methyl methacrylate) copolymers with benzoyl peroxide as an initiator. J. Appl. Polym. Sci. 2006, 101, 4083–4089. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Gu, J.; Sun, Y.; Ji, X. Cross-linking of poly (vinyl alcohol) with N, N′-methylene bisacrylamide via a radical reaction to prepare pervaporation membranes. RSC Adv. 2015, 5, 19859–19864. [Google Scholar] [CrossRef]
- Mahmood, A.; Sharif, A.; Muhammad, F.; Sarfraz, R.M.; Abrar, M.A.; Qaisar, M.N.; Anwer, N.; Amjad, M.W.; Zaman, M. Development and in vitro evaluation of (β-cyclodextrin-g-methacrylic acid)/Na+-montmorillonite nanocomposite hydrogels for controlled delivery of lovastatin. Int. J. Nanomed. 2019, 14, 5397–5413. [Google Scholar] [CrossRef] [Green Version]
- Badakhshanian, E.; Hemmati, K.; Ghaemy, M. Enhancement of mechanical properties of nanohydrogels based on natural gum with functionalized multiwall carbon nanotube: Study of swelling and drug release. Polymer 2016, 90, 282–289. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Sohail, M.; Sarfraz, R.M. β-cyclodextrin modification by cross-linking polymerization as highly porous nanomatrices for olanzapine solubility improvement; synthesis, characterization and bio-compatibility evaluation. J. Drug Deliv. Sci. Technol. 2022, 67, 102952. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Sohail, M.; Badshah, S.F.; Abdullah, O.; Khan, S.; Munir, A. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev. Ind. Pharm. 2021, 47, 465–476. [Google Scholar] [CrossRef]
- Faris, T.M.; Harisa, G.I.; Alanazi, F.K.; Samy, A.M.; Nasr, F.A. Developed simvastatin chitosan nanoparticles co-crosslinked with tripolyphosphate and chondroitin sulfate for ASGPR-mediated targeted HCC delivery with enhanced oral bioavailability. Saudi Pharm. J. 2020, 28, 1851–1867. [Google Scholar] [CrossRef]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Portero, A.; Remuñán-López, C.; Vila-Jato, J. Effect of chitosan and chitosan glutamate enhancing the dissolution properties of the poorly water soluble drug nifedipine. Int. J. Pharm. 1998, 175, 75–84. [Google Scholar] [CrossRef]
- Lim, L.M.; Tran, T.-T.; Wong, J.J.L.; Wang, D.; Cheow, W.S.; Hadinoto, K. Amorphous ternary nanoparticle complex of curcumin-chitosan-hypromellose exhibiting built-in solubility enhancement and physical stability of curcumin. Colloids Surf. B Biointerfaces 2018, 167, 483–491. [Google Scholar] [CrossRef]
- Saal, W.; Ross, A.; Wyttenbach, N.; Alsenz, J.; Kuentz, M. Unexpected Solubility Enhancement of Drug Bases in the Presence of a Dimethylaminoethyl Methacrylate Copolymer. Mol. Pharm. 2018, 15, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Alam, B.M.; Aouak, T.; Alandis, N.M.; Alam, M.M. Synthesis, characterization, drug solubility enhancement, and drug release study of poly (methacrylic acid-graft-simvastatin). Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 229–241. [Google Scholar] [CrossRef]
- Shiraishi, S.; Arahira, M.; Imai, T.; Otagiri, M. Enhancement of dissolution rates of several drugs by low-molecular chitosan and alginate. Chem. Pharm. Bull. 1990, 38, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.; Ahmad, M.; Minhas, M.; Tulain, R.; Khalid, I.; Barkat, K.; Rashid, A. Toxicological evaluation of xanthan gum based hydrogel formulation in Wistar rats using single dose study. Acta Pol. Pharm.-Drug Res. 2020, 77, 353–360. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Khan, K.U.; Sohail, M.; Khalid, I. Functionalized pectin hydrogels by cross-linking with monomer: Synthesis, characterization, drug release and pectinase degradation studies. Polym. Bull. 2020, 77, 339–356. [Google Scholar]
- Rahman, N.A.; Abu Hanifah, S.; Mobarak, N.N.; Su’Ait, M.S.; Ahmad, A.; Shyuan, L.K.; Khoon, L.T. Synthesis and characterizations of o-nitrochitosan based biopolymer electrolyte for electrochemical devices. PLoS ONE 2019, 14, e0212066. [Google Scholar] [CrossRef]
- Zheng, X.; Yin, Y.; Jiang, W.; Xing, L.; Pu, J. Synthesis and Characterization of Low Molecular Weight Chitosan. BioResources 2015, 10, 2338–2349. [Google Scholar] [CrossRef] [Green Version]
- Affandi, M.M.M.; Tripathy, M.; Majeed, A.B.A.; Shah, S.A.A. Solubility enhancement of simvastatin by arginine: Thermodynamics, solute–solvent interactions, and spectral analysis. Drug Des. Dev. Ther. 2016, 10, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Pardeshi, P.M.; Mungray, A.A. Photo-polymerization as a new approach to fabricate the active layer of forward osmosis membrane. Sci. Rep. 2019, 9, 1937. [Google Scholar] [CrossRef]
- Lowman, A.; Morishita, M.; Kajita, M.; Nagai, T.; Peppas, N. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci. 1999, 88, 933–937. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Gangadharappa, H.V.; Veerapu, G.; Hani, U.; Alhamhoom, Y.; Alqahtani, A.; Moin, A. Characterization, Optimization, In Vitro and In Vivo Evaluation of Simvastatin Proliposomes, as a Drug Delivery. AAPS PharmSciTech 2020, 21, 129. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Snoeck, D.; Schröfl, C.; De Belie, N.; Klemm, A.J.; Ichimiya, K.; Moon, J.; Wyrzykowski, M.; Lura, P.; Toropovs, N.; et al. Testing superabsorbent polymer (SAP) sorption properties prior to implementation in concrete: Results of a RILEM Round-Robin Test. Mater. Struct. 2018, 51, 28. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kim, E.-J.; Seo, S.-W.; Choi, H.-K. Effect of poloxamer on the dissolution of felodipine and preparation of controlled release matrix tablets containing felodipine. Arch. Pharm. Res. 2008, 31, 1023–1028. [Google Scholar] [CrossRef]












| Sr. No. | Parameters | 0-Month | 3rd Month | 6th Month |
|---|---|---|---|---|
| 01 | Physical description | Light yellow color | No visible change in color | No visible change in color |
| 02 | Entrapment efficiency (%) | 92.30 ± 2.11 | 91.14 ± 2.03 | 90.32 ± 1.42 |
| 03 | FTIR analysis | Performed | No major shift in IR spectra | No major shift in IR spectra |
| 04 | Solubilization efficiency | Increased | No significant change | No significant change |
| Sr. No. | Formulation Code | Average Particle Size of Nanomatrices (nm) |
|---|---|---|
| 01 | ANC-1 | 221.6 ± 11.4 |
| 02 | ANC-2 | 224.7 ± 8.1 |
| 03 | ANC-3 | 239.5 ± 7.2 |
| 04 | ANC-4 | 231.8 ± 8.4 |
| 05 | ANC-5 | 227.8 ± 17.8 |
| 06 | ANC-6 | 241.6 ± 11.0 |
| 07 | ANC-7 | 246.1 ± 12.3 |
| Observations for Acute Oral Toxicity Study for Control and Nanomatrices Treated Rats | ||
|---|---|---|
| Parameters | Control Group I | Nanomatrices-Treated Group II |
| Water intake per animal per day (mL) | ||
| Before treatment | 31 ± 1.24 | 32 ± 1.43 |
| Day 1 | 29 ± 1.09 | 31 ± 1.18 |
| Day 7 | 35 ± 0.89 | 37 ± 1.09 |
| Day 14 | 39 ± 1.37 | 40 ± 1.43 |
| Food intake per animal per day (kg) | ||
| Before treatment | 14 ± 1.17 | 15 ± 0.96 |
| Day 1 | 16 ± 1.02 | 13 ± 1.11 |
| Day 7 | 15 ± 1.27 | 14 ± 0.99 |
| Day 14 | 16 ± 1.07 | 17 ± 1.15 |
| Weight of animal (kg) | ||
| Before treatment | 200 ± 0.76 | 209 ± 1.23 |
| Day 1 | 204 ± 1.10 | 213 ± 1.44 |
| Day 7 | 211 ± 0.98 | 217 ± 1.11 |
| Day 14 | 218 ± 1.14 | 225 ± 1.22 |
| General illness/eye irritation/dermal irritation, | No | No |
| Lacrimation/salivation, | No | No |
| Convulsions/hyperactivity | No | No |
| Plasma Biochemical Analysis | Control Group I | Test Group II |
|---|---|---|
| Red blood cells 106/µL | 8.60 ± 0.67 | 8.58 ± 0.88 |
| White blood cells 103/µL | 4.3 ± 0.23 | 4.4 ± 0.34 |
| Hemoglobin g/dL | 14.38 ± 1.21 | 14.22 ± 1.11 |
| Hematocrit % | 43.31 ± 0.55 | 43.49 ± 0.74 |
| Monocytes % | 2.17 ± 0.49 | 2.06 ± 0.55 |
| Lymphocytes % | 75.02 ± 0.71 | 75.54 ± 0.68 |
| Neutrophils % | 26.5 ± 0.54 | 26.01 ± 0.39 |
| MCH pg | 17.5 ± 0.72 | 17.9 ± 0.65 |
| MCV fL (µm3) | 53.4 ± 0.22 | 53.7 ± 0.26 |
| MCHC g/dL | 33.2 ± 0.41 | 32.8 ± 0.38 |
| Serum Biochemical Analysis | Control Group I | Test Group II |
| ALT (U/L) | 29.82 ± 0.34 | 30.66 ± 0.38 |
| ALP (U/L) | 120.21 ± 0.19 | 121.32 ± 0.22 |
| AST (U/L) | 106.94 ± 0.15 | 107.32 ± 0.18 |
| Creatinine (mg/dL) | 0.65 ± 0.29 | 0.67 ± 0.31 |
| Urea (mg/dL) | 15.51 ± 0.94 | 15.55 ± 0.87 |
| Cholesterol (mg/dL) | 59.58 ± 0.82 | 59.64 ± 0.88 |
| Triglyceride (mg/dL) | 72.59 ± 0.42 | 72.39 ± 0.50 |
| Glucose (mg/dL) | 109.59 ± 0.64 | 109.70 ± 0.57 |
| Sr. No. | Formulation Code | Chitosan (g) | MAA (mL) | MBA (g) | BPO (g) | %DL | %DEE |
|---|---|---|---|---|---|---|---|
| 01 | ANC-1 | 0.3 | 2 | 3 | 0.5 | 74.12 ± 2.03 | 82.04 ± 1.2 |
| 02 | ANC-2 | 0.4 | 2 | 3 | 0.5 | 78.45 ± 1.58 | 89.64 ± 1.54 |
| 03 | ANC-3 | 0.5 | 2 | 3 | 0.5 | 81.82 ± 2.35 | 85.17 ± 2.01 |
| 04 | ANC-4 | 0.4 | 3 | 3 | 0.5 | 86.84 ± 1.49 | 90.74 ± 1.68 |
| 05 | ANC-5 | 0.4 | 4 | 3 | 0.5 | 90.04 ± 2.13 | 92.30 ± 2.07 |
| 06 | ANC-6 | 0.4 | 2 | 3.5 | 0.5 | 72.65 ± 1.67 | 81.22 ± 2.01 |
| 07 | ANC-7 | 0.4 | 2 | 4 | 0.5 | 68.49 ± 2.02 | 71.40 ± 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, A.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U.; Abdullah, O. Highly Responsive Chitosan-Co-Poly (MAA) Nanomatrices through Cross-Linking Polymerization for Solubility Improvement. Gels 2022, 8, 196. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8030196
Saleem A, Akhtar N, Minhas MU, Mahmood A, Khan KU, Abdullah O. Highly Responsive Chitosan-Co-Poly (MAA) Nanomatrices through Cross-Linking Polymerization for Solubility Improvement. Gels. 2022; 8(3):196. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8030196
Chicago/Turabian StyleSaleem, Anam, Naveed Akhtar, Muhammad Usman Minhas, Arshad Mahmood, Kifayat Ullah Khan, and Orva Abdullah. 2022. "Highly Responsive Chitosan-Co-Poly (MAA) Nanomatrices through Cross-Linking Polymerization for Solubility Improvement" Gels 8, no. 3: 196. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8030196