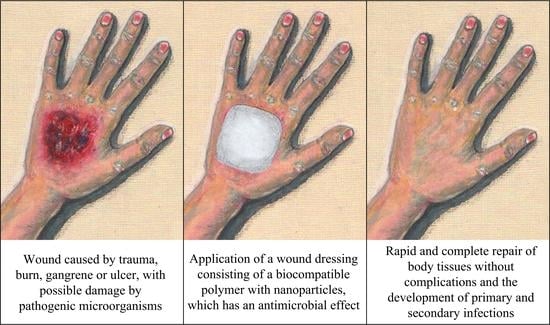
Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Nanoparticles of Metals
3.1.1. Silver Nanoparticles
3.1.2. Nanoparticles of Gold
3.1.3. Nanoparticles of Copper
3.2. Nanoparticles of Metal Oxides
3.2.1. Wound Dressings Containing Zinc Oxide Nanoparticles
3.2.2. Wound Dressings Containing Nanoparticles of Iron Oxides
3.2.3. Wound Dressings Containing Cerium Dioxide Nanoparticles
3.2.4. Wound Dressings Containing Titanium Dioxide Nanoparticles
3.2.5. Wound Dressings Containing Copper Oxide Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Varaprasad, K. Co-assembled ZnO (shell)—CuO (core) nano-oxide materials for microbial protection. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 74–80. [Google Scholar] [CrossRef]
- Varshney, S.; Nigam, A.; Pawar, S.J.; Mishra, N. An overview on biomedical applications of versatile silica nanoparticles, synthesized via several chemical and biological routes: A review. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 72–88. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric dental nanomaterials: Antimicrobial action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, A.K.; Koulaouzidou, E.A.; Gogos, C.; Achilias, D.S. Synthesis of novel dental nanocomposite resins by incorporating polymerizable, quaternary ammonium silane-modified silica nanoparticles. Polymers 2021, 13, 1682. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ozay, O. Synthesis of antibiotic-modified silica nanoparticles and their use as a controlled drug release system with antibacterial properties. Phosphorus Sulfur Silicon Relat. Elem. 2022, in press. [Google Scholar] [CrossRef]
- Razuvaeva, E.V.; Kalinin, K.T.; Sedush, N.G.; Nazarov, A.A.; Volkov, D.S.; Chvalun, S.N. Structure and cytotoxicity of biodegradable poly(d,l-lactide-co-glycolide) nanoparticles loaded with oxaliplatin. Mendeleev Commun. 2021, 31, 512–514. [Google Scholar] [CrossRef]
- Al Sawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-responsive nanocarriers in cancer therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Thao, N.T.; Wijerathna, H.M.S.M.; Kumar, R.S.; Choi, D.; Dananjaya, S.H.S.; Attanayake, A.P. Preparation and characterization of succinyl chitosan and succinyl chitosan nanoparticle film: In vitro and in vivo evaluation of wound healing activity. Int. J. Biol. Macromol. 2021, 193, 1823–1834. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Asghari, F.; Faridi-Majidi, R. Chitosan coated metallic nanoparticles with stability, antioxidant, and antibacterial properties: Potential for wound healing application. J. Appl. Polym. Sci. 2022, 139, 51766. [Google Scholar] [CrossRef]
- Deng, P.; Liang, X.; Chen, F.; Chen, Y.; Zhou, J. Novel multifunctional dual-dynamic-bonds crosslinked hydrogels for multi-strategy therapy of MRSA-infected wounds. Appl. Mater. Today 2022, 26, 101362. [Google Scholar] [CrossRef]
- Xie, T.; Ding, J.; Han, X.; Jia, H.; Yang, Y.; Liang, S.; Wang, W.; Liu, W.; Wang, W. Wound dressing change facilitated by spraying zinc ions. Mater. Horiz. 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Alven, S.; Buyana, B.; Feketshane, Z.; Aderibigbe, B.A. Electrospun nanofibers/nanofibrous scaffolds loaded with silver nanoparticles as effective antibacterial wound dressing materials. Pharmaceutics 2021, 13, 964. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, H.; Guo, B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nanomicro Lett. 2022, 14, 1–46. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.L.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, B.; Tian, Y.; Pang, L.; Xu, T.; Cong, H.; Shen, Y. Diversified antibacterial modification and latest applications of polysaccharide-based hydrogels for wound healthcare. Appl. Mater. Today 2022, 26, 101396. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, L.; Chen, Y.; Chen, G.; Zhao, T.; Yu, Y. Nano-silver functionalized polysaccharides as a platform for wound dressings: A review. Int. J. Biol. Macromol. 2022, 194, 644–653. [Google Scholar] [CrossRef]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Drabik, M.; Lipko, A.; Grzeczkowicz, A.; Stachowiak, R.; Marszalik, A.; Granicka, L.H. Composite Membrane Dressings System with Metallic Nanoparticles as an Antibacterial Factor in Wound Healing. Membranes 2022, 12, 215. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Massironi, A.; Franco, A.R.; Babo, P.S.; Puppi, D.; Chiellini, F.; Reis, R.L.; Gomes, M.E. Development and Characterization of Highly Stable Silver NanoParticles as Novel Potential Antimicrobial Agents for Wound Healing Hydrogels. Int. J. Mol. Sci. 2022, 23, 2161. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Pérez, C.J.; Desimone, M.F. Collagen Hydrogels Loaded with Silver Nanoparticles and Cannabis Sativa Oil. Antibiotics 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Nešović, K.; Mišković-Stanković, V. Silver/poly (vinyl alcohol)/graphene hydrogels for wound dressing applications: Understanding the mechanism of silver, antibacterial agent release. J. Vinyl Addit. Technol. 2022, 28, 196–210. [Google Scholar] [CrossRef]
- Santiago-Castillo, K.; Torres-Huerta, A.M.; del Ángel-López, D.; Domínguez-Crespo, M.A.; Dorantes-Rosales, H.; Palma-Ramírez, D.; Willcock, H. In Situ Growth of Silver Nanoparticles on Chitosan Matrix for the Synthesis of Hybrid Electrospun Fibers: Analysis of Microstructural and Mechanical Properties. Polymers 2022, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Mojally, M.; Sharmin, E.; Obaid, N.A.; Alhindi, Y.; Abdalla, A.N. Polyvinyl alcohol/corn starch/castor oil hydrogel films, loaded with silver nanoparticles biosynthesized in Mentha piperita leaves’ extract. J. King Saud Univ. Sci. 2022, 34, 101879. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Yang, X.; Zhang, W.; Jiang, M.; Wen, T.; Wang, J.; Guo, R.; Liu, H. Preparation and Application of Quaternized Chitosan- and AgNPs-Base Synergistic Antibacterial Hydrogel for Burn Wound Healing. Molecules 2021, 26, 4037. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Zhang, X.; Deng, Y.; Huang, D.; Huang, C.; Qu, Q. Quaternized chitin/tannic acid bilayers layer-by-layer deposited poly (lactic acid)/polyurethane nanofibrous mats decorated with photoresponsive complex and silver nanoparticles for antibacterial activity. Int. J. Biol. Macromol. 2022, 201, 448–457. [Google Scholar] [CrossRef]
- Bozkaya, O.; Arat, E.; Gök, Z.G.; Yiğitoğlu, M.; Vargel, İ. Production and characterization of hybrid nanofiber wound dressing containing Centella asiatica coated silver nanoparticles by mutual electrospinning method. Eur. Polym. J. 2022, 166, 111023. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608, 2278–2289. [Google Scholar] [CrossRef]
- Sethi, S.; Thakur, S.; Kaith, B.S.; Sharma, N.; Ansar, S.; Pandey, S.; Kuma, V. Biopolymer starch-gelatin embedded with silver nanoparticle–based hydrogel composites for antibacterial application. Biomass Convers. Biorefin. 2022, 1–22. [Google Scholar] [CrossRef]
- El-Hefnawy, M.E.; Alhayyani, S.; El-Sherbiny, M.M.; Sakran, M.I.; El-Newehy, M.H. Fabrication of Nanofibers Based on Hydroxypropyl Starch/Polyurethane Loaded with the Biosynthesized Silver Nanoparticles for the Treatment of Pathogenic Microbes in Wounds. Polymers 2022, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Madivoli, E.S.; Kareru, P.G.; Gachanja, A.N.; Makhanu, D.S.; Mugo, S.M. Cellulose-Based Hybrid Nanoarchitectonics with Silver Nanoparticles: Characterization and Antimicrobial Potency. J. Inorg. Organomet. Polym. Mater. 2022, 32, 854–863. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Zo, S.; Won, S.Y.; Kim, H.J.; Han, S.S. Injectable nanocomposite hydrogel as wound dressing agent with tunable multifunctional property. Mater. Lett. 2022, 307, 131062. [Google Scholar] [CrossRef]
- Yan, M.; Shi, J.; Tang, S.; Zhou, G.; Zeng, J.; Zhang, Y.; Zhang, H.; Yu, Y.; Guo, J. The construction of a seaweed-based antibacterial membrane loaded with nano-silver based on the formation of a dynamic united dual network structure. New J. Chem. 2022, 46, 511–520. [Google Scholar] [CrossRef]
- Chen, P.; Chai, M.; Mai, Z.; Liao, M.; Xie, X.; Lu, Z.; Zhang, W.; Zhao, H.; Dong, X.; Fu, X.; et al. Electrospinning polyacrylonitrile (PAN) based nanofibrous membranes synergic with plant antibacterial agent and silver nanoparticles (AgNPs) for potential wound dressing. Mater. Today Commun. 2022, 31, 103336. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Ahlberg, S.; Meinke, M.C.; Werner, L.; Epple, M.; Diendorf, J.; Blume-Peytavi, U.; Lademann, J.; Vogt, A.; Rancan, F. Comparison of silver nanoparticles stored under air or argon with respect to the induction of intracellular free radicals and toxic effects toward keratinocytes. Eur. J. Pharm. Biopharm. 2014, 88, 651–657. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, G. An Effective Wound Healing Material Based on Gold Incorporation into a Heparin-Polyvinyl Alcohol Nanocomposite: Enhanced In Vitro and In Vivo Care of Perioperative Period. J. Clust. Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.E.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Lemraski, E.G.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial double-layer wound dressing based on chitosan/polyvinyl alcohol/copper: In vitro and in vivo assessment. Int. J. Nanomed. 2021, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Byophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, M.; Chee, B.S.; Frassini, R.; Nugent, M.; Giovanela, M.; Roesch-Ely, M.; Crespo, J.d.S.; Devine, D.M. Antimicrobial PAA/PAH Electrospun Fiber Containing Green Synthesized Zinc Oxide Nanoparticles for Wound Healing. Materials 2021, 14, 2889. [Google Scholar] [CrossRef] [PubMed]
- Le, V.A.T.; Trinh, T.X.; Chien, P.N.; Giang, N.N.; Zhang, X.-R.; Nam, S.-Y.; Heo, C.-Y. Evaluation of the Performance of a ZnO-Nanoparticle-Coated Hydrocolloid Patch in Wound Healing. Polymers 2022, 14, 919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, H.; Hui, A.; Ding, J.; Liu, X.; Wang, A. Synergistic Effect of Glycyrrhizic Acid and ZnO/Palygorskite on Improving Chitosan-Based Films and Their Potential Application in Wound Healing. Polymers 2021, 13, 3878. [Google Scholar] [CrossRef]
- Kalemtas, A.; Kocer, H.B.; Aydin, A.; Terzioglu, P.; Aydin, G. Mechanical and antibacterial properties of ZnO/chitosan bio-composite films. J. Polym. Eng. 2022, 42, 35–47. [Google Scholar] [CrossRef]
- Hasanin, M.; Swielam, E.M.; Atwa, N.A.; Agwa, M.M. Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. Int. J. Biol. Macromol. 2022, 197, 121–130. [Google Scholar] [CrossRef]
- Joorabloo, A.; Khorasani, M.T.; Adeli, H.; Milan, P.B.; Amoupour, M. Using artificial neural network for design and development of PVA/chitosan/starch/heparinized nZnO hydrogels for enhanced wound healing. J. Ind. Eng. Chem. 2022, 108, 88–100. [Google Scholar] [CrossRef]
- Chen, F.C.; Huang, C.M.; Yu, X.W.; Chen, Y.Y. Effect of nano zinc oxide on proliferation and toxicity of human gingival cells. Hum. Exp. Toxicol. 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Keerthana, S.; Kumar, A. Potential risks and benefits of zinc oxide nanoparticles: A systematic review. Crit. Rev. Toxicol. 2020, 50, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Paydayesh, A.; Heleil, L.; Sh Dadkhah, A. Preparation and application of poly (hydroxyl ethyl methacrylate) nanocomposite hydrogels containing iron oxide nanoparticles as wound dressing. Polym. Compos. 2022, 30, 09673911211063106. [Google Scholar] [CrossRef]
- Moac, E.A.; Farcaş, C.; Coricovac, D.; Avram, S.; Mihali, C.V.; Drâghici, G.A.; Felicia, L.; Cornelia, P.; Dehelean, C. Oleic Acid Double Coated Fe3O4 Nanoparticles as Anti-Melanoma Compounds with a Complex Mechanism of Activity-In Vitro and In Ovo Assessment. J. Biomed. Nanotechnol. 2019, 15, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Zamani, K.; Allah-Bakhshi, N.; Akhavan, F.; Yousefi, M.; Golmoradi, R.; Ramezani, M.; Ramezani, F. Antibacterial effect of cerium oxide nanoparticle against Pseudomonas aeruginosa. BMC Biotechnol. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Xu, Y.; Sang, X.; Li, C.; Liu, Y.; Guo, Q.; Ramakrishna, S.; Wang, C.; Hu, P.; Nanda, H.S. PLLA-gelatin composite fiber membranes incorporated with functionalized CeNPs as a sustainable wound dressing substitute promoting skin regeneration and scar remodelling. J. Mater. Chem. B. 2022, 10, 1116–1127. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Bui, V.K.H.; Moon, J.Y.; Lee, Y.C. In-vitro cytotoxicity and oxidative stress induced by cerium aminoclay and cerium oxide nanoparticles in human skin keratinocyte cells. J. Nanosci. Nanotechnol. 2019, 19, 6369–6375. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Iordache, F.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Teodorescu, S.; et al. Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications. Nanomaterials 2022, 12, 239. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Toxicity assessment of six titanium dioxide nanoparticles in human epidermal keratinocytes. Cutan. Ocul. Toxicol. 2019, 38, 66–80. [Google Scholar] [CrossRef]
- Wright, C.; Iyer, A.K.V.; Wang, L.; Wu, N.; Yakisich, J.S.; Rojanasakul, Y.; Azad, N. Effects of titanium dioxide nanoparticles on human keratinocytes. Drug Chem. Toxicol. 2017, 40, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Karuppannan, S.K.; Ramalingam, R.; Khalith, S.M.; Musthafa, S.A.; Dowlath, M.J.H.; Munuswamy-Ramanujam, G.; Arunachalam, K.D. Copper oxide nanoparticles infused electrospun polycaprolactone/gelatin scaffold as an antibacterial wound dressing. Mater. Lett. 2021, 294, 129787. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nanofiber membranes for antimicrobial breath mask applications. Curr. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Yahyaei, B.; Manafi, S.; Fahimi, B.; Arabzadeh, S.; Pourali, P. Production of electrospun polyvinyl alcohol/microbial synthesized silver nanoparticles scaffold for the treatment of fungating wounds. Appl. Nanosci. 2018, 8, 417–426. [Google Scholar] [CrossRef]
- Allafchian, A.; Jalali, S.A.H.; Kabirzadeh, N. Characterisation and investigation of antibacterial properties of nylon 66/TPS/Ag NPs nanofibre membranes. Micro Nano Lett. 2018, 13, 1747–1751. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, S.M.; Mansur, L.L.; Ramos, C.P.; Lage, A.P.; Mansur, H.S. Physicochemical properties and antimicrobial activity of biocompatible carboxymethylcellulose-silver nanoparticle hybrids for wound dressing and epidermal repair. J. Appl. Polym. Sci. 2018, 135, 45812. [Google Scholar] [CrossRef]
- Chen, H.; Lan, G.; Ran, L.; Xiao, Y.; Yu, K.; Lu, B.; Dai, F.; Wu, D.; Lu, F. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 2018, 183, 70–80. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Lima, D.W.; de Oliveira, M.J.; Lugão, A.B.; Alcântara, M.T.; Devine, D.M.; de Sá, M.J. Synthesis and in vivo behavior of PVP/CMC/agar hydrogel membranes impregnated with silver nanoparticles for wound healing applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef]
- Ficai, D.; Ardelean, I.L.; Holban, A.M.; Ditu, L.M.; Gudovan, D.; Sönmez, M.A.R.I.A.; Trusca, R.; Kaya, A.; Ficai, A.; Andronescu, E. Manufacturing nanostructured chitosan-based 2D sheets with prolonged antimicrobial activity. Rom. J. Morphol. Embryol. 2018, 59, 517–525. [Google Scholar]
- Wang, Q.; Qian, Z.; Liu, B.; Liu, J.; Zhang, L.; Xu, J. In vitro and in vivo evaluation of new PRP antibacterial moisturizing dressings for infectious wound repair. J. Biomater. Sci. Polym. Ed. 2019, 30, 462–485. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Aktürk, A.; Taygun, M.E.; Güler, F.K.; Goller, G.; Küçükbayrak, S. Fabrication of antibacterial polyvinyl alcohol nanocomposite mats with soluble starch coated silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 255–262. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Silva-Bermudez, P.; Espana-Sanchez, B.L.; Luna-Hernández, E.; Almaguer-Flores, A.; Ibarra, C.; Garcia-Perez, V.I.; Velasquillo, C.; Luna-Barcenas, G. Fabrication and in vitro behavior of dual-function chitosan/silver nanocomposites for potential wound dressing applications. Mater. Sci. Eng. C 2019, 94, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A self-adapting hydrogel based on chitosan/oxidized konjac glucomannan/AgNPs for repairing irregular wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Sionkowska, A.; Walczak, M.; Michalska-Sionkowska, M. Preparation and characterization of collagen/chitosan composites with silver nanoparticles. Polym. Compos. 2020, 41, 951–957. [Google Scholar] [CrossRef]
- Preethi, G.U.; Unnikrishnan, B.S.; Sreekutty, J.; Archana, M.G.; Anupama, M.S.; Shiji, R.; Pillai, R.; Joseph, M.M.; Syama, H.P.; Sreelekha, T.T. Semi-interpenetrating nanosilver doped polysaccharide hydrogel scaffolds for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 142, 712–723. [Google Scholar] [CrossRef]
- Lekalakala, R.; Aderibigbe, B.A.; Owonubi, S.J.; Sadiku, E.R.; Fonkui, Y.T.; Ndinteh, D.T.; Ray, S.S. Gum Acacia/Carbopol-Based Biocomposites Loaded with Silver Nanoparticles as Potential Wound Dressings. Int. J. Nanosci. Nanotechnol. 2020, 16, 219–231. [Google Scholar]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.; Rannier Andrade, L.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Correa, C.B.; de Albuquerque Junior, R.L.; da Costa, L.P.; Shin, S.R.; et al. Silver nanoparticles-composing alginate/gelatine hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kaur, H. Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Ounkaew, A.; Kasemsiri, P.; Jetsrisuparb, K.; Uyama, H.; Hsu, Y.I.; Boonmars, T.; Artchayasawat, A.; Knijnenburg, J.T.; Chindaprasirt, P. Synthesis of nanocomposite hydrogel based carboxymethyl starch/polyvinyl alcohol/nanosilver for biomedical materials. Carbohydr. Polym. 2020, 248, 116767. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Synthesis, physical, mechanical and antibacterial properties of nanocomposites based on poly (vinyl alcohol)/graphene oxide–silver nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.X.; Dong, J.Y.; Li, Y.H.; Zhong, J.; Yu, H.; Yu, Q.Q.; Lei, M. Fabrication of Ag–ZnO@ carboxymethyl cellulose/K-carrageenan/graphene oxide/konjac glucomannan hydrogel for effective wound dressing in nursing care for diabetic foot ulcers. Appl. Nanosci. 2020, 10, 729–738. [Google Scholar] [CrossRef]
- Li, R.; Xu, Z.; Jiang, Q.; Zheng, Y.; Chen, Z.; Chen, X. Characterization and biological evaluation of a novel silver nanoparticle-loaded collagen-chitosan dressing. Regen. Biomater. 2020, 7, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Zepon, Κ.M.; Marques, M.S.; Hansen, A.W.; Pucci, C.D.; Morisso, F.D.; Ziulkoski, A.L.; do Nascimento, J.H.; Magnago, R.F.; Κanis, L.A. Polymer-based wafers containing in situ synthesized gold nanoparticles as a potential wound-dressing material. Mater. Sci. Eng. C 2020, 109, 110630. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ahmed, M.K. Wound healing activity of Chitosan/Polyvinyl Alcohol embedded by gold nanoparticles prepared by nanosecond laser ablation. J. Mol. Struct. 2020, 1217, 128401. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, Z.; Li, M.; Liu, L.; Luo, H.; Wang, P.; Zhang, D.; Yang, X.; Zhou, K.; Lei, S. Graphene oxide/copper nanoderivatives-modified chitosan/hyaluronic acid dressings for facilitating wound healing in infected full-thickness skin defects. Int. J. Nanomed. 2020, 15, 8231. [Google Scholar] [CrossRef]
- Carrillo-Rodríguez, J.C.; Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Padron, G.; Ledezma, A.; Betancourt-Galindo, R. Composite based on poly (acrylic acid-co-itaconic acid) hydrogel with antibacterial performance. Polym. Compos. 2018, 39, 171–180. [Google Scholar] [CrossRef]
- Patil, P.P.; Meshram, J.V.; Bohara, R.A.; Nanaware, S.G.; Pawar, S.H. ZnO nanoparticle-embedded silk fibroin–polyvinyl alcohol composite film: A potential dressing material for infected wounds. New J. Chem. 2018, 42, 14620–14629. [Google Scholar] [CrossRef]
- Swaroop, K.; Somashekarappa, H.M. In vitro biocompatibility and antibacterial activity of gamma ray crosslinked ZnO/PVA hydrogel nanocomposites. Mater. Today Proc. 2018, 5, 21314–21321. [Google Scholar] [CrossRef]
- Salama, A.H.M.E.D. Chitosan/Silk Fibroin/Zinc Oxide Nanocomposite as a Sustainable and Antimicrobial Biomaterial. Cell. Chem. Technol. 2018, 52, 903–907. [Google Scholar]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Dall, V.C.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Khalilipour, A.; Paydayesh, A. Characterization of polyvinyl alcohol/ZnO nanocomposite hydrogels for wound dressings. J. Macromol. Sci. Part B 2019, 58, 371–384. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Pozzolini, M.; Vicini, S.; Alloisio, M.; Castellano, M. Alginate-based electrospun membranes containing ZnO nanoparticles as potential wound healing patches: Biological, mechanical, and physicochemical characterization. ACS Appl. Mater. Interfaces 2019, 12, 3371–3381. [Google Scholar] [CrossRef]
- Nosrati, H.; Khodaei, M.; Banitalebi-Dehkordi, M.; Alizadeh, M.; Asadpour, S.; Sharifi, E.; Ai, J.; Soleimannejad, M. Preparation and characterization of poly (ethylene oxide)/zinc oxide nanofibrous scaffold for chronic wound healing applications. Polym. Med. 2020, 50, 41–51. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.E.A. Cerium oxide nanoparticle incorporated electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) membranes for diabetic wound healing applications. ACS Biomater. Sci. Eng. 2019, 6, 58–70. [Google Scholar] [CrossRef]
- Ulu, A.; Birhanlı, E.; Köytepe, S.; Ateş, B. Chitosan/polypropylene glycol hydrogel composite film designed with TiO2 nanoparticles: A promising scaffold of biomedical applications. Int. J. Biol. Macromol. 2020, 163, 529–540. [Google Scholar] [CrossRef]
- Hu, Y.; Ke, Q.; Li, Z.; Han, W.; Yan, Z. In situ synthesis of cuprous oxide/cellulose nanofibers gel and antibacterial properties. Comput. Mater. Contin. 2018, 56, 517–527. [Google Scholar]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M.; Kenny, J.M.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Kim, J.H. Preparation and properties of nylon 4/6 copolymer nanofibers containing silver-zeolite nanoparticles. Fiber. Polym. 2018, 19, 350–356. [Google Scholar] [CrossRef]
- Karimian, R.; Mehrabani, M.G.; Mehramuz, B.; Ganbarov, K.; Ejlali, L.; Tanomand, A.; Kamounah, F.S.; Rezaee, M.A.; Yoursefi, M.; Sheykhsaran, E.; et al. Poly (ε-Caprolactone)/cellulose nanofiber blend nanocomposites containing ZrO2 nanoparticles: A new biocompatible wound dressing bandage with antimicrobial activity. Adv. Pharm. Bull. 2020, 10, 577. [Google Scholar]
- Yudaev, P.A.; Maslennikova, V.V.; Konkova, A.A.; Butorova, I.A.; Chistyakov, E.M. Silver-containing hydrogel based on polyvinyl alcohol modified with nanoscale cyclotriphosphazene. Pub. Health Tox. 2021, 1, A23. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Waibel, K.H.; Haney, B.; Moore, M.; Whisman, B.; Gomez, R. Safety of chitosan bandages in shellfish allergic patients. Mil. Med. 2011, 176, 1153–1156. [Google Scholar] [CrossRef] [Green Version]



| Composition Code | PU Volume (mL) | HPS Volume (mL) | Water Dispersion AgNPs (mL) | Total Volume (mL) |
|---|---|---|---|---|
| AgNPs-0@NFs | 10 | 5 | 0 | 15 |
| AgNPs-1@NFs | 9 | 5 | 1 | 15 |
| AgNPs-2@NFs | 8 | 5 | 2 | 15 |
| AgNPs-3@NFs | 7 | 5 | 3 | 15 |
| Composition Code | ZOI Diameters (mm) | |||
|---|---|---|---|---|
| P. aeruginosa | E. faecalis | C. albicans | A. niger | |
| AgNPs-0@NFs | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| AgNPs-1@NFs | 15 ± 0.20 | 13 ± 0.18 | 10 ± 0.16 | 11 ± 0.23 |
| AgNPs-2@NFs | 21 ± 0.17 | 19 ± 0.12 | 17 ± 0.20 | 15 ± 0.15 |
| AgNPs-3@NFs | 26 ± 0.23 | 24 ± 0.25 | 23 ± 0.23 | 21 ± 0.17 |
| Ciprofloxacin | 12 ± 0.19 | 11 ± 0.25 | 8 ± 0.21 | 7 ± 0.14 |
| Nanofiller | The Studied Characteristics of the Wound Dressings | Ref. |
|---|---|---|
| AgNPs | Antimicrobial activity | [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] |
| Cytotoxicity analysis | [22,27,29,31,34,35] | |
| Biocompatibility | [22,28,30] | |
| Silver release measurements | [24,29,31,36] | |
| In vivo wound healing activity | [27,30] | |
| Swelling degree | [27,30,31] | |
| Porosity | [29,31] | |
| Mechanical properties | [25,28,29,31,35,36] | |
| Immunohistochemical analysis | [27,30] | |
| Water absorption | [29] | |
| Water vapor transmission rate | ||
| Air permeability | ||
| Hemocompatibility | [30,31] | |
| In vitro cell compatibility | [36] | |
| AuNPs | Antimicrobial activity | [39,41] |
| Cytotoxicity analysis | [41] | |
| Biocompatibility | [39] | |
| In vivo wound healing activity | [39] | |
| Swelling degree | [39] | |
| Mechanical properties | [39,41] | |
| CuNPs | Antimicrobial activity In vivo wound healing activity Copper ion release tesr | [42] |
| ZnO NPs | Antimicrobial activity | [44,47,48,49] |
| Cytotoxicity analysis | [49,50] | |
| In vivo wound healing activity | [45,48,49] | |
| Swelling degree | [46,49] | |
| Porosity | [49] | |
| Mechanical properties | [46,47] | |
| Water vapor transmission rate | [49] | |
| Hemocompatibility | [46] | |
| FeO NPs | Antimicrobial activity | [53] |
| Porosity | ||
| Water absorption | ||
| Iron release | ||
| Antidiabetic activity | ||
| Fe3O4 NPs | Antimicrobial activity | [54] |
| Cytotoxicity analysis | ||
| Biocompatibility | ||
| Swelling degree | ||
| Porosity | ||
| Mechanical properties | ||
| Water vapor transmission rate | ||
| CeO2 NPs | Antimicrobial activity | [56] |
| Cytotoxicity analysis | ||
| Evaluation of resistance genes expression in P. aeruginosa | ||
| In vivo wound healing activity | [57] | |
| Mechanical properties | ||
| Water absorption | ||
| In vitro cell proliferation test | ||
| MWCNT_TiO2 | Antimicrobial activity | [59] |
| Biocompatibility | ||
| Mechanical properties | ||
| CuO NPs | Mechanical properties Antimicrobial activity Biocompatibility | [62] |
| Nanofiller | Effect on Cells | Advantage (+) Disadvantage (−) |
|---|---|---|
| AgNPs | Oxidative stress. Superoxide and hydroxyl radical generation | Strong antibacterial action (+) May cause allergies (−) Toxic to human skin keratinocyte cells (−) |
| AuNPs | Presumably cause oxidative damage to bacteria | Nontoxic to human skin keratinocyte cells (+) Weak antimicrobial effect (−) |
| CuNPs | Presumably copper ions bind the DNA molecules of a bacterial cell | Nontoxic to human skin keratinocyte cells (+) Lower antimicrobial effect vs. antibiotics (−) |
| ZnO NPs | Electrostatic attraction of zinc ions to the bacterial cell membrane followed by release of the cell contents | Strong antibacterial action (+) Genotoxic to human epidermal cells (−) |
| FeO NPs, Fe3O4 NPs | Penetrate through cell membrane and prevent transmembrane electron trnsfer | Strong antibacterial action (+) Toxic to human skin keratinocyte cells (−) |
| CeO2 NPs | Oxidative stress on lipids and/or proteins in the plasma membrane through reduction in Ce4+ to Ce3+. | Strong antibacterial action (+) Low toxicity towards human skin keratinocyte cells (+) |
| TiO2 NPs | Oxidative stress. Generation of two reactive oxygen intermediates—OH and H2O2. | Nontoxic to human skin keratinocyte cells (+) Weak antimicrobial effect (−) |
| CuO NPs | Oxidative stress. Generation of four reactive oxygen intermediates—the superoxide oxygen radical, ·OH, H2O2, the singlet oxide. | Strong antibacterial action (+) Toxic to human skin keratinocyte cells (−) |
| Nanoparticle | Year | No. of Publications | References |
|---|---|---|---|
| AgNPs | 2018 | 6 | [65,66,67,68,69,70] |
| 2019 | 4 | [71,72,73,74] | |
| 2020 | 12 | [75,76,77,78,79,80,81,82,83,84,85,86] | |
| 2021 | 2 | [23,27] | |
| 2022 | 13 | [22,24,25,26,28,29,30,31,32,33,34,35,36] | |
| AuNPs | 2020 | 2 | [87,88] |
| 2021 | 2 | [39,41] | |
| CuNPs | 2020 | 1 | [89] |
| 2021 | 1 | [42] | |
| ZnO NPs | 2018 | 5 | [70,90,91,92,93] |
| 2019 | 4 | [94,95,96,97] | |
| 2020 | 2 | [85,98] | |
| 2021 | 2 | [44,46] | |
| 2022 | 5 | [45,47,48,49,50] | |
| FeO NPs | 2021 | 1 | [53] |
| Fe3O4 NPs | 2022 | 1 | [54] |
| CeO2 NPs | 2019 | 1 | [99] |
| 2021 | 1 | [56] | |
| 2022 | 1 | [57] | |
| TiO2 NPs | 2020 | 1 | [100] |
| 2022 | 1 | [59] | |
| CuO NPs | 2021 | 1 | [62] |
| Cu2O NPs | 2018 | 1 | [101] |
| Lignin NPs | 2018 | 1 | [102] |
| Silver zeolite NPs | 2018 | 1 | [103] |
| ZrO2 NPs | 2020 | 1 | [104] |
| Polymer | Year | No. of Publications | References |
|---|---|---|---|
| Chitosan and derivatives thereof | 2018 | 3 | [70,93,102] |
| 2019 | 4 | [71,72,74,94] | |
| 2020 | 8 | [75,76,81,82,86,88,89,100] | |
| 2021 | 4 | [27,42,46,53] | |
| 2022 | 6 | [25,34,35,47,48,49] | |
| Polyvinyl alcohol | 2018 | 4 | [65,91,92,102] |
| 2019 | 3 | [73,94,96] | |
| 2020 | 5 | [81,83,84,87,88] | |
| 2021 | 3 | [39,42,53] | |
| 2022 | 4 | [24,25,26,49] | |
| Cellulose and derivatives thereof | 2018 | 3 | [67,69,101] |
| 2020 | 2 | [85,104] | |
| 2022 | 2 | [33,59] | |
| Polycaprolactone | 2020 | 1 | [104] |
| 2021 | 3 | [41,56,62] | |
| 2022 | 1 | [29] | |
| Gelatin | 2020 | 1 | [80] |
| 2021 | 2 | [56,62] | |
| 2022 | 3 | [30,31,57] | |
| Starch and derivatives thereof | 2020 | 1 | [83] |
| 2022 | 3 | [31,32,49] | |
| Konjac glucomannan | 2018 | 1 | [68] |
| 2020 | 3 | [75,82,85] | |
| Collagen | 2020 | 2 | [76,86] |
| 2022 | 2 | [23,59] | |
| Silk fibroin | 2018 | 2 | [91,93] |
| 2019 | 1 | [71] | |
| Sodium alginate and calcium alginate | 2019 | 1 | [97] |
| 2020 | 1 | [80] | |
| 2022 | 1 | [35] | |
| Hyaluronic acid and derivatives thereof | 2020 | 1 | [89] |
| 2021 | 1 | [27] | |
| 2022 | 1 | [22] | |
| Polyalkylene glycols | 2018 | 1 | [69] |
| 2019 | 1 | [72] | |
| 2020 | 2 | [98,100] | |
| 2022 | 1 | [29] | |
| Keratin | 2019 | 1 | [95] |
| 2020 | 1 | [79] | |
| Polylactic acid | 2022 | 2 | [28,57] |
| κ-carrageenan | 2020 | 2 | [85,87] |
| Polyurethane | 2022 | 2 | [28,32] |
| Oxidized dextran | 2021 | 1 | [27] |
| Polyvinylpyrrolidone | 2018 | 1 | [69] |
| Agar | 2018 | 1 | [69] |
| Poly(acrylic acid-co-itaconic acid) | 2018 | 1 | [90] |
| Nylon 66 | 2018 | 1 | [66] |
| Nylon 4/6 copolymer | 2018 | 1 | [103] |
| Galacto-xyloglucan | 2020 | 1 | [77] |
| Gum acacia and carbopol | 2020 | 1 | [78] |
| HBV | 2019 | 1 | [99] |
| Polyacrylonitrile | 2022 | 1 | [36] |
| Heparin | 2021 | 1 | [39] |
| Polyacrylic acid and polyallylamine hydrochloride | 2021 | [44] | |
| Glycogen | 2022 | 1 | [48] |
| Polyhydroxyethyl methacrylate | 2022 | 1 | [54] |
| Quaternized chitin | 2022 | 1 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060329
Yudaev P, Mezhuev Y, Chistyakov E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels. 2022; 8(6):329. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060329
Chicago/Turabian StyleYudaev, Pavel, Yaroslav Mezhuev, and Evgeniy Chistyakov. 2022. "Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects" Gels 8, no. 6: 329. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060329