Gelatin Nanoparticles for Targeted Dual Drug Release out of Alginate-di-Aldehyde-Gelatin Gels
Abstract
:1. Introduction
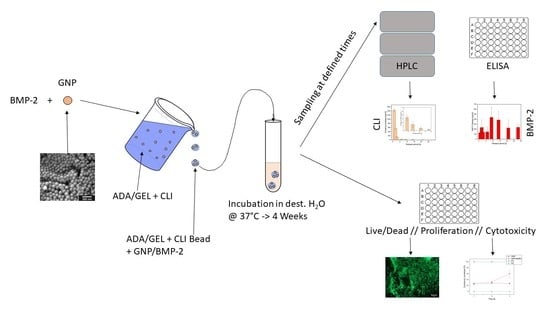
2. Results
2.1. Manufacturing Process of GNPs
2.2. Inclusion Capacity
2.3. Biocompatibility
2.3.1. Live Dead Staining
2.3.2. Cell Proliferation Assay (WST-I)
2.3.3. Lactate Dehydrogenase (LDH)
2.4. Drug Release Experiments
2.4.1. Clindamycin Release out of ADA-GEL Beads
2.4.2. FITC-Protein a Release out of GNPs within ADA-GEL Beads
2.4.3. BMP-2 Release out of GNPs within ADA-GEL Beads
3. Discussion
3.1. Characterization of GNP
3.2. Inclusion Capacity
3.3. Biocompatibility
3.4. Drug Release Experiments
4. Conclusions
5. Materials and Methods
5.1. Reagents and Materials
5.2. Manufacturing of the Gelatin Nanoparticles (GNPs)
Manufacturing of FITC-Protein A-Loaded GNPs and BMP-2 Loaded GNPs
5.3. Characterization of the GNP
5.3.1. Characterization of GNP by ESEM
5.3.2. Determination of the Inclusion Capacity
5.4. Biocompatibility
5.4.1. Live/Dead Assay
5.4.2. Cell Proliferation Assay
5.4.3. Lactate Dehydrogenase (LDH) Assay
5.5. ADA-GEL Hydrogel
5.5.1. Preparation of Alginate-di-Aldehyde (ADA)
5.5.2. Crosslinking ADA and GEL
5.6. ADA-GEL Beads with Drugs
5.6.1. ADA-GEL Beads with Clindamycin and FITC-Protein A-Containing GNPs
5.6.2. ADA-GEL Beads with CLI and BMP-2 Containing GNP
5.7. Drug Release Experiments
5.7.1. Drug Release from ADA-GEL Beads
5.7.2. Dual Drug Release from ADA-GEL Beads
5.7.3. Quantitative Analysis
5.7.4. Kinetics Model
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Tiemann, A.; Hugo, H. Osteomyelitis. Orthopädie Unf. Up2date 2020, 15, 67–82. [Google Scholar] [CrossRef]
- Schmelz, A.; Kinzl, L.; Einsiedel, T. Osteitis. Infections of the locomotive system. Unfallchirurg 2007, 110, 1039–1058. [Google Scholar] [CrossRef] [PubMed]
- Suedkamp, N.P.; Barbey, N.; Veuskens, A.; Tempka, A.; Haas, N.P.; Hoffmann, R.; Tscherne, H. The incidence of osteitis in open fractures: An analysis of 948 open fractures (a review of the hannover experience). J. Orthop. Trauma 1993, 7, 473–482. [Google Scholar] [CrossRef]
- Zimmerli, W.; Ryu, S.Y.; Patel, R.; Landersdorfer, C.B.; Bulitta, J.B.; Soergel, F.; Richards, R.; Moriarty, F. Bone and Joint Infections from Microbiology to Diagnostics and Treatment; John Wiley & Sons Inc.: Oxford, UK, 2015; ISBN 978-1-118-58177-3. [Google Scholar]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Wengler, A.; Nimptsch, U.; Mansky, T. Hip and knee replacement in germany and the USA. Dtsch. Arztebl. Int. 2014, 111, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Farid, Y.; Schettino, M.; Kapila, A.K.; Hamdi, M.; Cuylits, N.; Wauthy, P.; Ortiz, S. Decrease in surgical activity in the COVID-19 pandemic: An economic crisis. Br. J. Surg. 2020, 107, e300. [Google Scholar] [CrossRef]
- Destatis. Gesundheit—Fallpauschalenbezogene Krankenhausstatistik (Drg-Statistik) Operationen und Prozeduren der Vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller); Statistisches Bundesamt (Destatis): Wiesbaden, Germany, 2020. [Google Scholar]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of total joint replacement in the united states: Future projections to 2020–2040 using the national inpatient sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Steckelberg, J.M.; Osmon, D.R. Prosthetic joint infections. In Infections Associated with Indwelling Medical Devices; ASM Press: Washington, DC, USA, 2000; pp. 173–209. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Hamarat Sanlıer, S.; Yasa, M.; Cihnioglu, A.O.; Abdulhayoglu, M.; Yılmaz, H.; Ak, G. Development of gemcitabine-adsorbed magnetic gelatin nanoparticles for targeted drug delivery in lung cancer. Artif. Cells Nanomed. Biotechnol. 2016, 44, 943–949. [Google Scholar] [CrossRef]
- Coester, C.J.; Langer, K.; Von Briesen, H.; Kreuter, J. Gelatin nanoparticles by two step desolvation a new preparation method, surface modifications and cell uptake. J. Microencaps. 2000, 17, 187–193. [Google Scholar]
- Renner, L.; Perka, C.; Trampuz, A.; Renz, N. Treatment of periprosthetic infections. Chirurg 2016, 87, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Path. 2018, 14, e1007112. [Google Scholar] [CrossRef] [Green Version]
- Kayser, F.H.; Böttger, E.C. Medizinische Mikrobiologie; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2005; ISBN 978-3-13-1514431. [Google Scholar]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and enzymatically dual cross-linked oxidized alginate gelatin hydrogels with tunable stiffness and degradation behavior for tissue engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Klemm. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin. Microbiol. Infect. 2000, 7, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Diez-Pena, E.; Frutos, G.; Frutos, P.; Barrales-Rienda, J.M. Gentamicin sulphate release from a modified commercial acrylic surgical radiopaque bone cement. I. Influence of the gentamicin concentration on the release process mechanism. Chem. Pharm. Bull. 2002, 50, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X. Gelatin Nanoparticles & Nanocrystals for Dermal Delivery. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2014. [Google Scholar]
- Azizian, S.; Hadjizadeh, A.; Niknejad, H. Chitosan-gelatin porous scaffold incorporated with chitosan nanoparticles for growth factor delivery in tissue engineering. Carbohydr. Polym. 2018, 202, 315–322. [Google Scholar] [CrossRef]
- Reeves, D.S.; Holt, H.A.; Phillips, I.; King, A.; Miles, R.S.; Paton, R.; Wise, R.; Andrews, J.M. Activity of clindamycin against staphylococcus aureus and staphylococcus epidermidis from four uk centres. J. Antimicrob. Chemother. 1991, 27, 469–474. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release ii. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Hathout, R.M.; Metwally, A.A. Gelatin nanoparticles. In Pharmaceutical Nanotechnology: Basic Protocols; Weissig, V., Elbayoumi, T., Eds.; Springer: New York, NY, USA, 2019; pp. 71–78. [Google Scholar]
- Modaresifar, K.; Hadjizadeh, A.; Niknejad, H. Design and fabrication of gelma/chitosan nanoparticles composite hydrogel for angiogenic growth factor delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Dai, H.; Ma, L.; Yu, Y.; Tang, M.; Li, Y.; Hu, W.; Liu, T.; Zhang, Y. Food-grade gelatin nanoparticles: Preparation, characterization, and preliminary application for stabilizing pickering emulsions. Foods 2019, 8, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable chitosan nanoparticle coatings on titanium for the delivery of bmp-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Yhee, J.Y.; Kim, S.H.; Kwon, I.C.; Kim, K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized sirna in tumor-bearing mice. J. Control. Release 2013, 172, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Geena, M.G.; Lakshmi, H.; Koyakutty, M.; Nair, S.; Menon, D. Poly-(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti-inflammatory drug ibuprofen-sodium: An in vitro and in vivo analysis. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.O.; Gómez-Benito, M.J.; Folgado, J.; Fernandes, P.R.; García-Aznar, J.M. In silico mechano-chemical model of bone healing for the regeneration of critical defects: The effect of bmp-2. PLoS ONE 2015, 10, e0127722. [Google Scholar] [CrossRef] [Green Version]
- Kissling, S.; Seidenstuecker, M.; Pilz, I.H.; Suedkamp, N.P.; Mayr, H.O.; Bernstein, A. Sustained release of rhbmp-2 from microporous tricalciumphosphate using hydrogels as a carrier. BMC Biotechnol. 2016, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, K.; Duan, L.; Fu, T.; Li, J.; Mu, Z.; Wang, S.; Zou, Q.; Chen, L.; Feng, Y.; et al. Strontium ranelate incorporated enzyme-cross-linked gelatin nanoparticle/silk fibroin aerogel for osteogenesis in ovx-induced osteoporosis. ACS Biomater. Sci. Eng. 2019, 5, 1440–1451. [Google Scholar] [CrossRef]
- Abdelrady, H.; Hathout, R.M.; Osman, R.; Saleem, I.; Mortada, N.D. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur. J. Pharm. Sci. 2019, 133, 115–126. [Google Scholar] [CrossRef]
- Minardi, S.; Fernandez-Moure, J.S.; Fan, D.; Murphy, M.B.; Yazdi, I.K.; Liu, X.; Weiner, B.K.; Tasciotti, E. Biocompatible plga-mesoporous silicon microspheres for the controlled release of bmp-2 for bone augmentation. Pharmaceutics 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-S.; Yang, S.-S.; Kim, C.S. Incorporation of bmp-2 nanoparticles on the surface of a 3d-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 170, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-L.; Chen, K.-H.; Su, W.-Y.; Lee, Y.-H.; Wu, C.-C.; Lin, F.-H. Cationic gelatin nanoparticles for drug delivery to the ocular surface: In vitro and in vivo evaluation. J. Nanomater. 2013, 2013, 238351. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.-T.; Huang, J.-Y.; Chen, M.-H.; Chen, C.-Y.; Shyong, Y.-J.; Yen, K.-C.; Sun, Y.-J.; Ke, C.-J.; Cheng, Y.-H.; Lin, F.-H. Development of gelatin nanoparticles conjugated with phytohemagglutinin erythroagglutinating loaded with gemcitabine for inducing apoptosis in non-small cell lung cancer cells. J. Mater. Chem. B 2016, 4, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Seidenstuecker, M.; Ruehe, J.; Suedkamp, N.P.; Serr, A.; Wittmer, A.; Bohner, M.; Bernstein, A.; Mayr, H.O. Composite material consisting of microporous β-tcp ceramic and alginate for delayed release of antibiotics. Acta Biomater. 2017, 51, 433–446. [Google Scholar] [CrossRef]
- Kuehling, T.; Schilling, P.; Bernstein, A.; Mayr, H.O.; Serr, A.; Wittmer, A.; Bohner, M.; Seidenstuecker, M. A human bone infection organ model for biomaterial research. Acta Biomater. 2022, 144, 230–241. [Google Scholar] [CrossRef]
- Sarker, B.; Rompf, J.; Silva, R.; Lang, N.; Detsch, R.; Kaschta, J.; Fabry, B.; Boccaccini, A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int. J. Biol. Macromol. 2015, 78, 72–78. [Google Scholar] [CrossRef]
- Neovius, E.; Lemberger, M.; Docherty Skogh, A.C.; Hilborn, J.; Engstrand, T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.; Kaschta, J.; Detsch, R.; Schubert, D.; Cicha, I.; Boccaccini, A. Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [Green Version]
- Batzias, G.C.; Delis, G.A.; Koutsoviti-Papadopoulou, M. A new hplc/uv method for the determination of clindamycin in dog blood serum. J. Pharm. Biomed. Anal. 2004, 35, 545–554. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release i. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrade, S.; Ritschl, L.; Süss, R.; Schilling, P.; Seidenstuecker, M. Gelatin Nanoparticles for Targeted Dual Drug Release out of Alginate-di-Aldehyde-Gelatin Gels. Gels 2022, 8, 365. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060365
Schrade S, Ritschl L, Süss R, Schilling P, Seidenstuecker M. Gelatin Nanoparticles for Targeted Dual Drug Release out of Alginate-di-Aldehyde-Gelatin Gels. Gels. 2022; 8(6):365. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060365
Chicago/Turabian StyleSchrade, Sophie, Lucas Ritschl, Regine Süss, Pia Schilling, and Michael Seidenstuecker. 2022. "Gelatin Nanoparticles for Targeted Dual Drug Release out of Alginate-di-Aldehyde-Gelatin Gels" Gels 8, no. 6: 365. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060365