Smart Antifreeze Hydrogels with Abundant Hydrogen Bonding for Conductive Flexible Sensors
Abstract
:1. Introduction
2. Results and Discussion
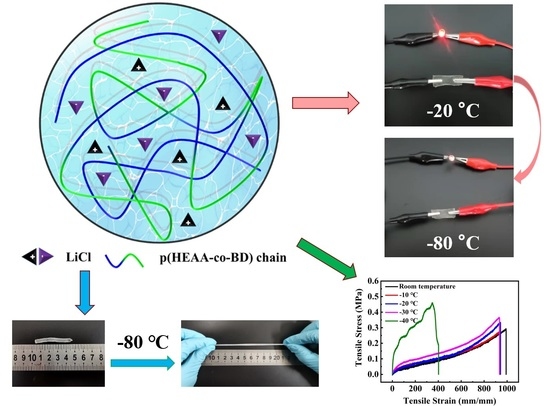
2.1. Preparation of LiCl/p(HEAA−co−BD) Antifreeze Conductive Hydrogel
2.2. Comparison of Anti−Freezing Capacity of Different Hydrogel Components
2.3. Low−Temperature Tensile Properties of LiCl/p(HEAA−co−BD) Anti−Freezing Conductive Hydrogels
2.4. Adhesion of LiCl/p(HEAA−co−BD) Hydrogel
2.5. Conductivity of LiCl/p(HEAA−co−BD) Antifreeze Hydrogels
2.6. Sensing Performance of LiCl/p(HEAA−co−BD) Hydrogel
3. Conclusions
4. Experimental Parts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, J.; Huang, J.; Zhou, G.; Liu, S. Versatile fabrication of ordered cellular structures double network composite hydrogel and application for cadmium removal. J. Chem. Thermodyn. 2020, 141, 105918. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Wu, J. Conductive hydrogel−and organohydrogel−based stretchable sensors. ACS Appl. Mater. Interfaces 2021, 13, 2128–2144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly stretchable, self−adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Yang, C.; Su, F.; Xu, Y.; Ma, Y.; Tang, L.; Zhou, N.; Liang, E.; Wang, G.; Tang, J. pH Oscillator−Driven Jellyfish−like Hydrogel Actuator with Dissipative Synergy between Deformation and Fluorescence Color Change. ACS Macro Lett. 2022, 11, 347–353. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, D.; Gong, L.; Zhang, Y.; Xie, S.; Ren, B.; Liu, Y.; Yang, F.; Zhou, G.; Chang, Y. Double−network physical cross−linking strategy to promote bulk mechanical and surface adhesive properties of hydrogels. Macromolecules 2019, 52, 9512–9525. [Google Scholar] [CrossRef]
- Chakraborty, P.; Oved, H.; Bychenko, D.; Yao, Y.; Tang, Y.; Zilberzwige-Tal, S.; Wei, G.; Dvir, T.; Gazit, E. Nanoengineered Peptide-Based Antimicrobial Conductive Supramolecular Biomaterial for Cardiac Tissue Engineering. Adv. Mater. 2021, 33, 2008715. [Google Scholar] [CrossRef]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive hydrogels—A novel material: Recent advances and future perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.S.; Guan, J.; Wu, S.J. Processing, mechanical properties and bio−applications of silk fibroin−based high−strength hydrogels. Acta Biomater. 2021, 125, 57–71. [Google Scholar] [CrossRef]
- Dong, Y.; Zhuang, H.; Hao, Y.; Zhang, L.; Yang, Q.; Liu, Y.; Qi, C.; Wang, S. Poly (N−isopropyl−acrylamide)/poly (γ−glutamic acid) thermo−sensitive hydrogels loaded with superoxide dismutase for wound dressing application. Int. J. Nanomed. 2020, 15, 1939–1950. [Google Scholar] [CrossRef] [Green Version]
- Rowland, M.J.; Parkins, C.C.; McAbee, J.H.; Kolb, A.K.; Hein, R.; Loh, X.J.; Watts, C.; Scherman, O.A. An adherent tissue−inspired hydrogel delivery vehicle utilised in primary human glioma models. Biomaterials 2018, 179, 199–208. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Guo, Z.; Tang, Z.; Luo, Y.; Xie, S.; Li, N.; Xu, J. Flexible Li+/agar/pHEAA double−network conductive hydrogels with self−adhesive and self−repairing properties as strain sensors for human motion monitoring. React. Funct. Polym. 2021, 168, 105054. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Han, X.; Dai, L.; Li, C.; Wang, J.; Zhong, Y.; Yu, F.; Si, C. Conductive cellulose nanofibrils−reinforced hydrogels with synergetic strength, toughness, self−adhesion, flexibility and adjustable strain responsiveness. Carbohydr. Polym. 2020, 250, 117010. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tang, L.; Xu, Y.; Yao, J.; Tang, G.; Dai, B.; Wang, W.; Tang, J.; Gong, L. A self−powered flexible sensing system based on a super−tough, high ionic conductivity supercapacitor and a rapid self−recovering fully physically crosslinked double network hydrogel. J. Mater. Chem. C 2022, 10, 3027–3035. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Xu, Y.; Li, Y.; Dai, B.; Yang, C.; Liu, A.; Tang, J.; Gong, L. Design of a DNA-Based Double Network Hydrogel for Electronic Skin Applications. Adv. Mater. Technol. 2022, 7, 2200066. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A review of conductive hydrogel used in flexible strain sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost−effective and ultrasensitive detection of Amaranth. Colloids Surf. B Biointerfaces 2018, 172, 565–572. [Google Scholar] [CrossRef]
- Alam, A.; Meng, Q.; Shi, G.; Arabi, S.; Ma, J.; Zhao, N.; Kuan, H.C. Electrically conductive, mechanically robust, pH−sensitive graphene/polymer composite hydrogels. Compos. Sci. Technol. 2016, 127, 119–126. [Google Scholar] [CrossRef]
- Xiang, K.; Wen, X.; Hu, J.; Wang, S.; Chen, H. Rational Fabrication of Nitrogen and Sulfur Codoped Carbon Nanotubes/MoS2 for High-Performance Lithium–Sulfur Batteries. ChemSusChem 2019, 12, 3602–3614. [Google Scholar] [CrossRef]
- Hsiao, L.-Y.; Jing, L.; Li, K.; Yang, H.; Li, Y.; Chen, P.-Y. Carbon nanotube−integrated conductive hydrogels as multifunctional robotic skin. Carbon 2020, 161, 784–793. [Google Scholar] [CrossRef]
- Sun, M.; Wu, X.; Liu, C.; Xie, Z.; Deng, X.; Zhang, W.; Huang, Q.; Huang, B. The In Situ grown of activated Fe−N−C nanofibers derived from polypyrrole on carbon paper and its electro−catalytic activity for oxygen reduction reaction. J. Solid State Electrochem. 2018, 22, 1217–1226. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Li, Y.; Wang, X.; Yang, W.; Ren, J. Polypyrrole−Doped Conductive Self−Healing Composite Hydrogels with High Toughness and Stretchability. Biomacromolecules 2021, 22, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, L.; Lu, Y.; Hu, C.; Liang, Z.; Long, L.; Ning, N.; Chen, J.; Guo, Y.; Yang, Z.; et al. Intrinsic Antibacterial and Conductive Hydrogels Based on the Distinct Bactericidal Effect of Polyaniline for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 52308–52320. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gong, L.; Xu, Y.; Wu, S.; Wang, W.; Zheng, B.; Tang, Y.; Zhang, D.; Tang, J.; Zheng, J. Mechanically Strong Metal–Organic Framework Nanoparticle−Based Double Network Hydrogels for Fluorescence Imaging. ACS Appl. Nano Mater. 2022, 5, 1348–1355. [Google Scholar] [CrossRef]
- Xu, H.; Lv, Y.; Qiu, D.; Zhou, Y.; Zeng, H.; Chu, Y. An ultra−stretchable, highly sensitive and biocompatible capacitive strain sensor from an ionic nanocomposite for on−skin monitoring. Nanoscale 2019, 11, 1570–1578. [Google Scholar] [CrossRef]
- Kang, T.H.; Chang, H.; Choi, D.; Kim, S.; Moon, J.; Lim, J.A.; Lee, K.Y.; Yi, H. Hydrogel−templated transfer−printing of conductive nanonetworks for wearable sensors on topographic flexible substrates. Nano Lett. 2019, 19, 3684–3691. [Google Scholar] [CrossRef]
- Peng, H.; Xin, Y.; Xu, J.; Liu, H.; Zhang, J. Ultra−stretchable hydrogels with reactive liquid metals as asymmetric force−sensors. Mater. Horiz. 2019, 6, 618–625. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra−stretchable wearable strain sensors based on skin−inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Cai, C.; Li, Q.; Wen, C.; Zhang, X.; Wang, X.; Yang, J.; Zhang, L. Ionic conductive hydrogels with long−lasting antifreezing, water retention and self−regeneration abilities. Chem. Eng. J. 2021, 419, 129478. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.J.A.M. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Gu, K.; Yao, J.; Shao, Z.; Chen, X. Poly (vinyl alcohol) Hydrogels with Integrated Toughness, Conductivity, and Freezing Tolerance Based on Ionic Liquid/Water Binary Solvent Systems. ACS Appl. Mater. Interfaces 2021, 13, 29008–29020. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Chen, P.; Zhu, Y.; Wen, C.; Gao, Y.; Yang, J.; Zhang, X.; Zhang, L. Zwitterionic osmolyte-based hydrogels with antifreezing property, high conductivity, and stable flexibility at subzero temperature. Adv. Funct. Mater. 2020, 30, 1907986. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Xu, Y.; Cui, T.; Li, Y.; Wang, W.; Gong, L.; Tang, J. High toughness fully physical cross−linked double network organohydrogels for strain sensors with anti−freezing and anti−fatigue properties. Mater. Adv. 2021, 2, 6655–6664. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Lin, Y.J.; Enriquez, E.; Peng, X.F.; Turng, L.S. Highly stretchable and biocompatible strain sensors based on mussel−inspired super−adhesive self−healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef] [PubMed]







| LiCl/p(HEAA−co−BD)/H2O | LiCl/pHEAA/H2O | LiCl/pHEAA/H2O | pHEAA/H2O | |
|---|---|---|---|---|
| Monomeric ratios (g:g) | 0.45:8:2:5 | 0.45:8:5 | 8:2:5 | 8:5 |
| Pre−polymerized liquid |  |  |  |  |
| Post−aggregation |  |  |  |  |
| −20 ℃ |  |  |  |  |
| −80 ℃ |  |  |  |  |
| Glass transition temperature | −85.6 °C | −51.8 °C | −64.9 °C | −17.1 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, B.; Cui, T.; Xu, Y.; Wu, S.; Li, Y.; Wang, W.; Liu, S.; Tang, J.; Tang, L. Smart Antifreeze Hydrogels with Abundant Hydrogen Bonding for Conductive Flexible Sensors. Gels 2022, 8, 374. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060374
Dai B, Cui T, Xu Y, Wu S, Li Y, Wang W, Liu S, Tang J, Tang L. Smart Antifreeze Hydrogels with Abundant Hydrogen Bonding for Conductive Flexible Sensors. Gels. 2022; 8(6):374. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060374
Chicago/Turabian StyleDai, Bailin, Ting Cui, Yue Xu, Shaoji Wu, Youwei Li, Wu Wang, Sihua Liu, Jianxin Tang, and Li Tang. 2022. "Smart Antifreeze Hydrogels with Abundant Hydrogen Bonding for Conductive Flexible Sensors" Gels 8, no. 6: 374. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060374