Evaluation of the Accessibility of Molecules in Hydrogels Using a Scale of Spin Probes
Abstract
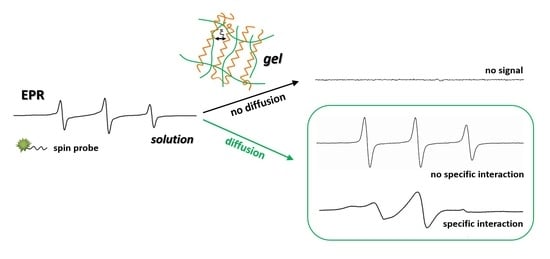
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Appel, E.A.; del Bario, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Ladewig, K.; Fu, Q.; Blencowe, A.; Qiao, G.G. Cyclodextrin-based supramolecular assemblies and hydrogels: Recent advances and future perspectives. Macromol. Rapid Commun. 2014, 35, 1166–1184. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, C.P.; Brenner, M.P.; Ribbeck, K. Enhanced diffusion by binding to the crosslinks of a polymer gel. Nat. Commun. 2018, 9, 4348. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, L.; Wang, Y.C.; Li, J.; Nie, J.; Wang, Z.L. A flexible multifunctional triboelectric nanogenerator based on MXene/PVA hydrogel. Adv. Funct. Mater. 2021, 31, 2104928. [Google Scholar] [CrossRef]
- Li, S.N.; Yu, Z.R.; Guo, B.F.; Guo, K.Y.; Li, Y.; Gong, L.X.; Zhao, L.; Bae, J.; Tang, L.C. Environmentally stable, mechanically flexible, self-adhesive, and electrically conductive Ti3C2TX MXene hydrogels for wide-temperature strain sensing. Nano Energy 2021, 90, 106502. [Google Scholar] [CrossRef]
- Li, S.N.; Li, B.; Yu, Z.R.; Gong, L.X.; Xia, Q.Q.; Feng, Y.; Jia, D.; Zhou, Y.; Tang, L.C. Chitosan in-situ grafted magnetite nanoparticles toward mechanically robust and electrically conductive ionic-covalent nanocomposite hydrogels with sensitive strain-responsive resistance. Compos. Sci. Technol. 2020, 195, 108173. [Google Scholar] [CrossRef]
- Kirchhof, S.; Abrami, M.; Messmann, V.; Hammer, N.; Goepferich, A.M.; Grassi, M.; Brandl, F.P. Diels-Alder hydrogels for controlled antibody release: Correlation between mesh size and release rate. Mol. Pharm. 2015, 12, 3358–3368. [Google Scholar] [CrossRef]
- Ionita, G. Characterisation and tailoring the properties of hydrogels using spectroscopic methods. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Bag, D.S.; Alam, S. Chiral chemical absorption property of a cross-linked poly(N-isopropyl acrylamide-co-sodium acrylate) thermoresponsive smart gel. Chirality 2012, 24, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Javid, N.; Sefcik, J.; Halling, P.J.; Ulijn, R.V. Salt-induced control of supramolecular order in biocatalytic hydrogelation. Langmuir 2012, 28, 16664–16670. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Kuang, Y.; Gao, Y.; Zhang, Y.; Gao, P.; Xu, B. Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels. J. Am. Chem. Soc. 2010, 132, 2719–2728. [Google Scholar] [CrossRef]
- Vesković, A.; Nakarada, Đ.; Popović Bijelić, A. A novel methodology for hydrogel water content determination by EPR: The basis for real-time monitoring of controlled drug release and hydrogel swelling and degradation. Polym. Test. 2021, 98, 107187. [Google Scholar] [CrossRef]
- Ionita, G.; Ariciu, A.M.; Turcu, O.; Chechik, V. Properties of polyethylene glycol/cyclodextrin hydrogels revealed by spin probe and spin labelling methods. Soft Matter 2014, 10, 1778–1783. [Google Scholar] [CrossRef]
- Hinderberg, D. EPR spectroscopy in polymer science. In EPR Spectroscopy; Topics in Current Chemistry; Drescher, M., Jeschke, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 321, pp. 67–90. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S.; Salikhov, K.M. Foundations of Modern EPR; World Scientific: Singapore, 1998. [Google Scholar]
- Ionita, G.; Caragheorgheopol, A.; Caldararu, H.; Jones, L.; Chechik, V. Inclusion complexes of cyclodextrins with nitroxide-based spin probes in aqueous solutions. Org. Biomol. Chem. 2009, 7, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Ionita, G.; Chechik, V. Mobility of spin probes in viscous cyclodextrin solutions. Phys. Chem. Chem. Phys. 2010, 12, 6956–6960. [Google Scholar] [CrossRef] [PubMed]
- Ionita, G.; Mocanu, S.; Matei, I. Conformational preferences of TEMPO type radicals in complexes with cyclodextrins revealed by a combination of EPR spectroscopy, induced circular dichroism and molecular modeling. Phys. Chem. Chem. Phys. 2020, 22, 12154–12165. [Google Scholar] [CrossRef]
- Mocanu, S.; Matei, I.; Ionescu, S.; Tecuceanu, V.; Marinescu, G.; Ionita, P.; Culita, D.; Leonties, A.; Ionita, G. Complexation of β-cyclodextrin with dual molecular probes bearing fluorescent and paramagnetic moieties linked by short polyether chains. Phys. Chem. Chem. Phys. 2017, 19, 27839–27847. [Google Scholar] [CrossRef]
- Mocanu, S.; Matei, I.; Leonties, A.; Tecuceanu, V.; Hanganu, A.; Minea, Z.; Stancu, A.; Popescu, E.I.; Ionita, G. New flexible molecular probes bearing dansyl and TEMPO moieties for host–guest interactions in solution and gels. New J. Chem. 2019, 43, 11233–11240. [Google Scholar] [CrossRef]
- Matei, I.; Ariciu, A.M.; Neacsu, M.V.; Collauto, A.; Salifoglou, A.; Ionita, G. Cationic spin probe reporting on thermal denaturation and complexation–decomplexation of BSA with SDS. Potential applications in protein purification processes. J. Phys. Chem. B 2014, 118, 11238–11252. [Google Scholar] [CrossRef]
- Bruce, C.D.; Berkowitz, M.L.; Perera, L.; Forbes, M.D. Molecular dynamics simulation of sodium dodecyl sulfate micelle in water: Micellar structural characteristics and counterion distribution. J. Phys. Chem. B 2002, 106, 3788–3793. [Google Scholar] [CrossRef]
- Tojo, A.; Kinugasa, S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int. J. Nephrol. 2012, 2012, 481520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionita, G.; Marinescu, G.; Ilie, C.; Anghel, D.F.; Smith, D.K.; Chechik, V. Sorption of metal ions by poly(ethylene glycol)/β-CD hydrogels leads to gel-embedded metal nanoparticles. Langmuir 2013, 29, 9173–9178. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.; Bem, M.; Leonties, A.R.; Radutiu, C.; Popescu, E.I.; Mocanu, S.; Savonea, F.; Baratoiu, R.; Ionita, G. Processes mediated by gold nanoparticles encapsulated in polymeric gels evidenced by EPR spectroscopy. Rev. Roum. Chim. 2021, 66, 295–302. [Google Scholar] [CrossRef]
- Peters, T., Jr. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, R.; Zou, L.; McClements, D.J. Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability, protein retention and protein release. Food Hydrocoll. 2016, 58, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.H.E.; Lundberg, D.; Ribeiro, A.J.; Veiga, F.J.; Miguel, M.G.; Lindman, B.; Olsson, U. Encapsulation of DNA in macroscopic and nanosized calcium alginate gel particles. Langmuir 2013, 29, 15926–15935. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell encapsulation within alginate microcapsules: Immunological challenges and outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [Green Version]
- Cesteros, L.C.; Ramirez, C.A.; Pecina, A.; Katime, I. Poly(ethylene glycol-β-cyclodextrin) gels: Synthesis and properties. J. Appl. Polym. Sci. 2006, 102, 1162–1166. [Google Scholar] [CrossRef]
- Cesteros, L.C.; Ramirez, C.A.; Pecina, A.; Katime, I. Synthesis and properties of hydrophilic networks based on poly(ethylene glycol) and β-cyclodextrin. Macromol. Chem. Phys. 2007, 208, 1764–1772. [Google Scholar] [CrossRef]
- Caragheorgheopol, A.; Caldararu, H.; Dragutan, I.; Joela, H.; Brown, W. Micellization and micellar structure of a poly(ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) triblock copolymer in water solution, as studied by the spin probe technique. Langmuir 1997, 13, 6912–6921. [Google Scholar] [CrossRef]
- Feix, J.B.; Bachowski, G.J.; Girotti, A.W. Photodynamic action of Merocyanine 540 on erythrocyte membranes: Structural perturbation of lipid and protein constituents. Biochim. Biophys. Acta 1991, 1075, 28–35. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, I.; Ariciu, A.-M.; Popescu, E.I.; Mocanu, S.; Neculae, A.V.F.; Savonea, F.; Ionita, G. Evaluation of the Accessibility of Molecules in Hydrogels Using a Scale of Spin Probes. Gels 2022, 8, 428. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8070428
Matei I, Ariciu A-M, Popescu EI, Mocanu S, Neculae AVF, Savonea F, Ionita G. Evaluation of the Accessibility of Molecules in Hydrogels Using a Scale of Spin Probes. Gels. 2022; 8(7):428. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8070428
Chicago/Turabian StyleMatei, Iulia, Ana-Maria Ariciu, Elena Irina Popescu, Sorin Mocanu, Alexandru Vincentiu Florian Neculae, Florenta Savonea, and Gabriela Ionita. 2022. "Evaluation of the Accessibility of Molecules in Hydrogels Using a Scale of Spin Probes" Gels 8, no. 7: 428. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8070428