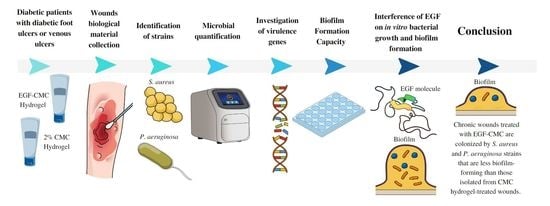
Effectiveness of Epidermal Growth Factor Loaded Carboxymethylcellulose (EGF-CMC) Hydrogel in Biofilm Formation in Wounds of Diabetic Patients: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Results
2.1. Health History and Wound Characteristics
2.2. Identification of S. aureus and P. aeruginosa Strains
2.3. Antimicrobial Susceptibility
2.4. Microbial Load
2.5. Biofilm Formation Assays
2.6. Identification of Virulence Genes
2.7. Interference of EGF in In Vitro Bacterial Growth and Biofilm Formation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Study Design and Population
5.3. Study Procedures
5.4. Identification of S. aureus and P. aeruginosa Strains and Antimicrobial Susceptibility Tests
5.5. Microbial Quantification Using Quantitative Real-Time Polymerase Chain Reaction (qPCR)
5.6. Biofilm Formation Capacity
5.7. Investigation of Virulence Genes
5.8. Interference of EGF in In Vitro Bacterial Growth and Biofilm Formation
5.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Meaning | Page |
| EGF | Epidermal growth factor | 1 |
| EGF-CMC | Epidermal growth factor loaded carboxymethylcellulose gel | 1 |
| S. aureus | Staphylococcus aureus | 1 |
| P. aeruginosa | Pseudomonas aeruginosa | 1 |
| CMC | Carboxymethylcellulose | 1 |
| ECM | Extracellular matrix | 2 |
| -CH2-COOH | Carboxymethyl group | 2 |
| FGF | Fibroblast growth factor | 2 |
| VEGF | Vascular endothelial growth factor | 2 |
| PDGF | Platelet-derived growth factor | 2 |
| EPS | Extracellular polysaccharide matrix | 3 |
| F | Absolute frequency | 3 |
| RF | Relative frequency | 3 |
| ABI | Ankle–brachial index | 3 |
| PAD | Peripheral obstructive arterial disease. | 3 |
| MRSA | Methicillin-resistant Staphylococcus aureus | 5 |
| qPCR | Quantitative real-time polymerase chain reaction | 5 |
| W1 | First week | 5 |
| W6 | Sixth week | 5 |
| W12 | Twelfth week | 5 |
| ExoS | Exoenzyme S gene | 7 |
| ExoU | Exoenzyme U gene | 7 |
| OD620 | Optical density measured in 620 nanometers | 8 |
| OD570 | Optical density measured in 570 nanometers | 8 |
| PRP | Platelet-rich plasma | 9 |
| rhEFG | Recombinant human epidermal growth factor | 11 |
| ReBEC UTN | Brazilian Registry of Clinical Trials code | 11 |
| mL | Milliliter | 11 |
| NaCl | Sodium chloride | 12 |
| TSB | Trypticase soy broth | 12 |
| MALDI-TOF MS | Matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry (MS) | 12 |
| CLSI | Clinical and Laboratory Standards Institute | 12 |
| µg | Microgram | 12 |
| ATCC | American Type Culture Collection | 12 |
| PCR | Polymerase chain reaction | 12 |
| bp | Base pairs | 12 |
| DNA | Deoxyribonucleic acid | 12 |
| Ct | Threshold cycle | 13 |
| PBS | Phosphate-buffered saline | 13 |
| SPSS | Statistical Package for the Social Sciences software | 13 |
| RR | Relative risk | 14 |
References
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Khan, Z.Z.; Labala, R.K.; Ahmad, J.; Noor, S.; Mishra, B.K. Financial burden of diabetic foot ulcers to world: A progressive topic to discuss always. Ther. Adv. Endocrinol. Metab. 2018, 9, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Swiaôtoniowska, N.; Sarzynska, K.; Szymanska-Chabowska, A.; Jankowska-Polanska, B. The role of education in type 2 diabetes treatment. Diabetes Res. Clin. Pract. 2019, 151, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Reardon, R.; Simring, D.; Kim, B.; Mortensen, J.; Williams, D.; Leslie, A. The diabetic foot ulcer. Aust. J. Gen. Pract. 2020, 49, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient angiogenesis-based diabetic wound healing/Skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. Hydrogel—Based biopolymers for regenerative medicine applications: A critical review. Int. J. Polym. Mater. 2022, 71, 155–172. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic hydrogels to promote wound healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Oliveira, B.C.; Oliveira, B.G.R.B.; Deutsch, G.; Pessanha, F.S.; Castilho, S.R. Effectiveness of a synthetic human recombinant epidermal growth factor in diabetic patients wound healing: Pilot, double-blind, randomized clinical controlled trial. Wound Repair. Regen. 2021, 29, 920–926. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-One: Multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small 2022, 18, e2104229. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Camacho-Rodríguez, H.; Mendoza-Marí, Y.; Falcón-Cama, V.; García-Ojalvo, A.; Herrera-Martínez, L.; Guillén-Nieto, G. Epidermal growth factor in healing diabetic foot ulcers: From gene expression to tissue healing and systemic biomarker circulation. MEDICC Rev. 2020, 22, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Techapichetvanich, T.; Wanitphakdeedecha, R.; Iamphonrat, T.; Phothong, W.; Eimpunth, S.; Hidajat, I.J.; Manuskiatti, W. The effects of recombinant human epidermal growth factor containing ointment on wound healing and post inflammatory hyperpigmentation prevention after fractional ablative skin resurfacing: A split-face randomized controlled study. J. Cosmet. Dermatol. 2018, 17, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Han, S.H.; Hong, J.P.; Han, S.K.; Lee, D.H.; Kim, B.S.; Ahn, J.H.; Lee, J.W. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res. Clin. Pract. 2018, 142, 335–344. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Z.; Huang, Y.; Wang, B.; Yang, P.; Fan, Y.; Hou, A.; Yang, B.; Zhao, Z.; Quan, G.; et al. In situ gelation of rhEGF-containing liquid crystalline precursor with good cargo stability and system mechanical properties: A novel delivery system for chronic wounds treatment. Biomater. Sci. 2019, 7, 995–1010. [Google Scholar] [CrossRef]
- Oliveira, B.G.R.B.; Oliveira, B.C.; Deutsch, G.; Pessanha, F.S.; Thiré, R.M.S.M.; Castilho, S.R. rhEGF-loaded hydrogel in the treatment of chronic wounds in patients with diabetes: Clinical cases. Gels. 2022, 8, 523. [Google Scholar] [CrossRef]
- International Wound Infection Institute (IWII). Wound Infection in Clinical Practice. Wounds Int. 2022, 1, 1–57. [Google Scholar]
- Oliveira, F.P.; Pires, B.M.F.B.; Silva, K.C.F.A.; Carvalho, B.T.F.; Teixeira, L.A.; Paula, G.R.; Oliveira, B.G.R.B. Prevalence, antimicrobial susceptibility, and clonal diversity of Pseudomonas aeruginosa in chronic wounds. J. Wound Ostomy Cont. Nurs. 2017, 44, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.M.F.B.; Oliveira, F.P.; Oliveira, B.G.R.B.; Fuly, P.S.C.F.; Ferreira-Carvalho, B.T.; Paula, G.R.; Teixeira, L.A. Monitoring and molecular characterization of Staphylococcus aureus isolated from chronic wounds. Adv. Skin Wound Care. 2018, 31, 399–405. [Google Scholar] [CrossRef]
- Pires, B.M.F.B.; Oliveira, B.G.R.B.; Bokehi, L.C.; Luiz, R.R.; Carvalho, B.T.F.; Santana, R.F.; Souza, P.A.; Paula, G.R.; Teixeira, L.A. Clinical and microbiological outcomes associated with use of platelet-rich plasma in chronic venous leg uclers: A randomized controlled trial. J. Wound Ostomy Cont. Nurs. 2021, 48, 292–299. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm management in wound care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; You, S.; Gao, T.; Lan, Y.; Deng, H.; Li, Z.P.; Hu, R.; Shen, J. All-in-one: Harnessing multifunctional injectable natural hydrogels for ordered therapy of bacteria-infected diabetic wounds. J. Chem. Eng. 2022, 439, 135691. [Google Scholar] [CrossRef]
- Oliveira, B.G.R.B.; Oliveira, F.P.; Teixeira, L.A.; Paula, G.R.; Oliveira, B.C.; Pires, B.M.F.B. Epidermal growth factor vs platelet-rich plasma: Activity against chronic wound microbiota. Int. Wound J. 2019, 16, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.I.M.; Fregoso, D.R.; Gallegos, A.; Yoon, D.J.; Fuentes, J.J.; Crawford, R.; Kaba, H.; Yang, H.; Isseroff, R.R. Beta adrenergic receptor antagonist can modify Pseudomonas aeruginosa biofilm formation in vitro: Implications for chronic wounds. FASEB J. 2022, 36, e22057. [Google Scholar] [CrossRef]
- Neopane, P.; Nepal, H.P.; Shrestha, R.; Uehara, O.; Abiko, Y. In vitro biofilm formation by Staphylococcus aureus isolated from wounds of hospital-admitted patients and their association with antimicrobial resistance. Int. J. Gen. Med. 2018, 11, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumaran, M.S. Platelet-rich plasma: The journey so far! Indian Dermatol. Online J. 2020, 11, 685–992. [Google Scholar] [CrossRef] [PubMed]
- Bahamondez-Canas, T.F.; Heersema, L.A.; Smyth, H.D.C. Current status of in vitro models and assays for susceptibility testing for wound biofilm infections. Biomedicines 2019, 30, 34. [Google Scholar] [CrossRef]
- Ertugrul, B.M.; Lipsky, B.A.; Ture, M.; Sakarya, S. Risk factors for infection with Pseudomonas aeruginosa in diabetic foot infections. J. Am. Podiatr. Med. Assoc. 2017, 107, 483–489. [Google Scholar] [CrossRef]
- Mandapalli, P.K.; Labala, S.; Jose, A.; Bhatnagar, S.; Janupally, R.; Sriram, D.; Venuganti, V.V.K. Layer-by-layer thin films for co-delivery of tgf-β sirna and epidermal growth factor to improve excisional wound healing. AAPS Pharm. Sci. Tech. 2017, 18, 809–820. [Google Scholar] [CrossRef]
- Gabadage, K.; Chirino-Trejo, M.; Campbell, J.; Luby, C. Efficacy of recombinant bovine epidermal growth factor in the treatment of experimental subclinical Staphylococcus aureus mastitis in a ewe model. Vet. Rec. Open 2017, 4, e000179. [Google Scholar] [CrossRef]
- Melendez, J.H.; Frankel, Y.M.; An, A.T.; Williams, L.; Price, L.B.; Wang, N.Y.; Lazarus, G.S.; Zenilman, J.M. Real-time PCR assays compared to culture-based approaches for identification of aerobic bacteria in chronic wounds. Clin. Microbiol. Infect. 2010, 16, 1762–1769. [Google Scholar] [CrossRef]
- Akram, A.; Izhar, M.; Lal, C.; Ghaffar, H.; Zafar, S.; Saifullah, A.; Yaseen, A. Frequency of panton valentine leucocidin gene in Staphylococcus aureus from skin and soft tissue infections. J. Ayub Med. Coll. Abbottabad. 2020, 32, 487–491. [Google Scholar]
- Khodayary, R.; Nikokar, I.; Mobayen, M.R.; Afrasiabi, F.; Araghian, A.; Elmi, A.; Moradzadeh, M. High incidence of type III secretion system associated virulence factors (exoenzymes) in Pseudomonas aeruginosa isolated from iranian burn patients. BMC Res. Notes 2019, 12, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Oliveira, B.G.R.B.; Castilho, S.R.; Futuro, D.O. Safety and efficiency of carboximethylcellulose hydrogel 2% in leg ulcers. Rev. Pesqui. (Univ. Fed. Estado Rio J.) 2013, 5, 690–695. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Murthy, K.N.C. Challenges in healing wound: Role of complementary and alternative medicine. Front. Nutr. 2022, 8, 791899. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.S.; Lindberg, R.B.; Mason, A.D.; Pruitt, B.A.; Colonel, M.C. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wound. J. Trauma 1976, 16, 84–94. [Google Scholar] [CrossRef]
- Mahnic, A.; Breznik, V.; Ihan, M.B.; Rupnik, M. Comparison between cultivation and sequencing based approaches for microbiota analysis in swabs and biopsies of chronic wounds. Front. Med. 2021, 8, 607255. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100; CLSI: Malvern, UK, 2020. [Google Scholar]
- Choi, H.J.; Kim, M.H.; Cho, M.S.; Kim, B.K.; Kim, J.Y.; Kim, C.; Park, D.S. Improved PCR for identification of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2013, 97, 3643–3651. [Google Scholar] [CrossRef]
- Tan, T.Y.; Corden, S.; Barnes, R.; Cookson, B. Rapid identification of methicillin-resistant Staphylococcus aureus from positive blood cultures by real-time fluorescence PCR. J. Clin. Microbiol. 2001, 39, 4529–4531. [Google Scholar] [CrossRef] [PubMed]
- Jabalameli, F.; Mirsalehian, A.; Khoramian, B.; Aligholi, M.; Khoramrooz, S.S.; Asadollahi, P.; Taherikalani, M.; Emaneini, M. Evaluation of biofilm production and characterization of genes encoding type III secretion system among Pseudomonas aeruginosa isolated from burn patients. Burns 2012, 38, 1192–1197. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of panton-valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Gajdács, M.; Pallós, P.; Ónodi, B.; Stájer, A.; Matusovits, D.; Kárpáti, K.; Burián, K.; Battah, B.; Ferrari, M.; et al. Relationship between biofilm-formation, phenotypic virulence factors and antibiotic resistance in environmental Pseudomonas aeruginosa. Pathogens. 2022, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Medronho, R.A. Epidemiology; Editora Atheneu: São Paulo, Brazil, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI or the editor(s). MDPI or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |



| Variable | Global (n = 25) | EGF-CMC (n = 14) | CMC (n = 11) | p-Value * | |||
|---|---|---|---|---|---|---|---|
| F | RF | F | RF | F | RF | ||
| Gender | 0.656 (a) | ||||||
| Female | 7 | 28.0% | 3 | 21.4% | 4 | 36.4% | |
| Male | 18 | 72.0% | 11 | 78.6% | 7 | 63.6% | |
| Age (years) | 0.267 (b) | ||||||
| 46|―52 | 1 | 4.0% | 0 | 0.0% | 1 | 9.1% | |
| 52|―64 | 11 | 44.0% | 8 | 57.1% | 3 | 27.3% | |
| 64|―76 | 13 | 52.0% | 6 | 42.9% | 7 | 63.6% | |
| ABI classification | 0.536 (a) | ||||||
| PAD mild to moderate | 9 | 36.0% | 5 | 35.7% | 4 | 36.4% | |
| Normal | 16 | 64.0% | 9 | 64.3% | 7 | 63.6% | |
| Glycated Hemoglobin > 7% | 15 | 60.0% | 9 | 64.3% | 6 | 54.5% | 0.697 (b) |
| Injury type | 1.000 (a) | ||||||
| Diabetic | 17 | 68.0% | 9 | 64.3% | 8 | 72.7% | |
| Venous | 8 | 32.0% | 5 | 35.7% | 3 | 27.3% | |
| Injury area (cm2) | 0.727 (b) | ||||||
| 2.0|―12.0 | 16 | 64.0% | 8 | 57.1% | 8 | 72.7% | |
| 12.0|―|52.0 | 9 | 36.0% | 6 | 42.8% | 3 | 27.2% | |
| Exudate | 0.407 (a) | ||||||
| Serous | 18 | 72.0% | 9 | 64.3% | 9 | 81.8% | |
| Serosanguineous | 7 | 28.0% | 5 | 35.7% | 2 | 18.2% | |
| Exudate Amount | 0.572 (b) | ||||||
| Minimal | 8 | 32.0% | 3 | 21.4% | 5 | 45.5% | |
| Moderate | 11 | 44.0% | 8 | 57.1% | 3 | 27.3% | |
| Large | 6 | 24.0% | 3 | 21.4% | 3 | 27.3% | |
| Margin | 0.317 (b) | ||||||
| Epithelized | 15 | 60.0% | 7 | 50.0% | 8 | 72.7% | |
| Hyperkeratotic | 6 | 24.0% | 4 | 28.6% | 2 | 18.2% | |
| Maceration | 4 | 16.0% | 3 | 21.4% | 1 | 9.1% | |
| Granulation (% of bed that was covered) | 0.851 (b) | ||||||
| 1|―50 | 8 | 32.0% | 3 | 21.4% | 5 | 45.5% | |
| 51|―|100 | 17 | 68.0% | 11 | 78.6% | 6 | 54.6% | |
| Slough (% of bed that was covered) | 0.317 (b) | ||||||
| 0|―25 | 17 | 68.0% | 9 | 64.3% | 8 | 72.7% | |
| 26|―|100 | 8 | 32.0% | 5 | 35.7% | 3 | 27.3% | |
| Time of injury (months) | 0.809 (b) | ||||||
| Up to 6 months | 4 | 16.0% | 1 | 7.1% | 2 | 18.2% | |
| 7|―59 | 10 | 40.0% | 6 | 42.9% | 5 | 45.4% | |
| 60|―|480 | 11 | 44.0% | 7 | 50.0% | 4 | 36.4% | |
| Evaluation | EGF-CMC Group (n = 14) | CMC Group (n = 11) | Fisher’s Exact Test p-Value Comparing the Incidences in Both Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa | S. aureus | P. aeruginosa | S. aureus | |||||||
| Number of Cases | Incidence | Number of Cases | Incidence | Number of Cases | Incidence | Number of Cases | Incidence | P. aeruginosa | S. aureus | |
| Week 1 | 3 | 21.4% | 3 | 21.4% | 2 | 18.2% | 2 | 18.2% | 1.000 | 1.000 |
| Week 6 | 3 | 21.4% | 1 | 7.1% | 1 | 9.1% | 3 | 27.3% | 0.604 | 0.288 |
| Week 12 | 5 | 35.7% | 1 | 7.1% | 1 | 9.1% | 3 | 27.3% | 0.180 | 0.288 |
| Just one evaluation * | 8 | 57.1% | 4 | 28.6% | 3 | 27.3% | 6 | 54.5% | 0.227 | 0.241 |
| Assay | Primer Name | 5′-3′ Sequence | Size (bp) | References |
|---|---|---|---|---|
| Quantitative PCR of P. aeruginosa | PA-431-C-F | CTGGGTCGAAAGGTGGTTGTTATC | 232 | [39] |
| PA-431-C-R | GCGGCTGGTGCGGCTGAGTC | |||
| Virulence genes in P. aeruginosa strains | exoS-F | TCAGGTACCCGGCATTCACTACGCGG | 572 | [41] |
| exoS-R | TCACTGCAGGTTCGTGACGTCTTTCTTTTA | |||
| exoU-F | CCTTAGCCATCTCAACGGTAGTC | 911 | [41] | |
| exoU-R | GAGGGCGAAGCTGGGGAGGTA | |||
| Quantitative PCR of S. aureus | SA-442-F | TCGGTACACGATATTCTTCACA | 179 | [40] |
| SA-442-R | ACTCTCGTATGACAGCTTC | |||
| Virulence genes in S. aureus strains | lukS-PV F | GCATCAASTGTATTGGATAGCAAAAGC | 463 | [42] |
| lukF-PV R | ATCATTAGGTAAAATGTCTGGACATGATCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessanha, F.S.; Oliveira, B.G.R.B.d.; Oliveira, B.C.; Deutsch, G.; Teixeira, F.L.; Bokehi, L.C.; Calomino, M.A.; Rodrigues de Castilho, S.; Thiré, R.M.d.S.M.; Teixeira, L.A.; et al. Effectiveness of Epidermal Growth Factor Loaded Carboxymethylcellulose (EGF-CMC) Hydrogel in Biofilm Formation in Wounds of Diabetic Patients: A Randomized Clinical Trial. Gels 2023, 9, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9020117
Pessanha FS, Oliveira BGRBd, Oliveira BC, Deutsch G, Teixeira FL, Bokehi LC, Calomino MA, Rodrigues de Castilho S, Thiré RMdSM, Teixeira LA, et al. Effectiveness of Epidermal Growth Factor Loaded Carboxymethylcellulose (EGF-CMC) Hydrogel in Biofilm Formation in Wounds of Diabetic Patients: A Randomized Clinical Trial. Gels. 2023; 9(2):117. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9020117
Chicago/Turabian StylePessanha, Fernanda Soares, Beatriz Guitton Renaud Baptista de Oliveira, Bianca Campos Oliveira, Gabriela Deutsch, Felipe Lopes Teixeira, Luciana Castilho Bokehi, Mariana Alcântara Calomino, Selma Rodrigues de Castilho, Rossana Mara da Silva Moreira Thiré, Lenise Arneiro Teixeira, and et al. 2023. "Effectiveness of Epidermal Growth Factor Loaded Carboxymethylcellulose (EGF-CMC) Hydrogel in Biofilm Formation in Wounds of Diabetic Patients: A Randomized Clinical Trial" Gels 9, no. 2: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9020117