Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials
Abstract
:1. Introduction
2. Fundamental Aspects of the Anaerobic Digestion Process
3. Improving the Performance of the Anaerobic Digestion Process
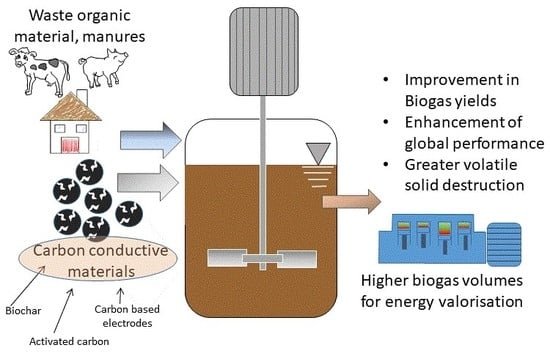
4. The Use of Carbon Conductive Materials for Improving Anaerobic Digestion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Okoh, A.I.; Makaka, G.; Simon, M. Microbial anaerobic digestion (bio-digesters) as an approach to the decontamination of animal wastes in pollution control and the generation of renewable energy. Int. J. Environ. Res. Public Health 2013, 10, 4390–4417. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. Regulatory Promotion and Benefit Analysis of Biogas-Power and Biogas-Digestate from Anaerobic Digestion in Taiwan’s Livestock Industry. Fermentation 2018, 4, 57. [Google Scholar] [CrossRef]
- Spinosa, L.; Ayol, A.; Baudez, J.C.; Canziani, R.; Jenicek, P.; Leonard, A.; Rulkens, W.; Xu, G.; Van Dijk, L. Sustainable and innovative solutions for sewage sludge management. Water 2011, 3, 702–717. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Pastor-Bueis, R.; Mulas, R.; Gómez, X.; González-Andrés, F. Innovative liquid formulation of digestates for producing a biofertilizer based on Bacillus siamensis: Field testing on sweet pepper. J. Plant Nutr. Soil Sci. 2017, 180, 748–758. [Google Scholar] [CrossRef]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A mathematical model for dynamic simulation of anaerobic digestion of complex substrates: Focusing on ammonia inhibition. Biotechnol. Bioeng. 1993, 42, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2005, 40, 3412–3418. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenerg. 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Pullammanappallil, P.C.; Chynoweth, D.P.; Lyberatos, G.; Svoronos, S.A. Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of swine manure and crude glycerine: Increasing glycerine ratio results in preferential degradation of labile compounds. Water Air Soil Pollut. 2016. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Petousi, I.; Manios, T. Co-digestion of sewage sludge with glycerol to boost biogas production. Waste Manag. 2010, 30, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Batstone, D.J. Anaerobic digestion: Process. Solid Waste Technol. Manag. 2010. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Ingram-Smith, C. Methanosaeta, the forgotten methanogen? Trends Microbiol. 2007, 15, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.E.; Rotaru, C.; Lovley, D.R. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2011, 2, e00159-11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonmati, A.; Flotats, X.; Mateu, L.; Campos, E. Study of thermal hydrolysis as a pretreatment to mesophilic anaerobic digestion of pig slurry. Water Sci. Technol. 2001, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Rosas, J.G.; Morán, A.; Gómez, X. Effect of ultrasound pretreatment on sludge digestion and dewatering characteristics: Application of particle size analysis. Water 2015, 7, 6483–6495. [Google Scholar] [CrossRef]
- Sträuber, H.; Bühligen, F.; Kleinsteuber, S.; Nikolausz, M.; Porsch, K. Improved anaerobic fermentation of wheat straw by alkaline pre-treatment and addition of alkali-tolerant microorganisms. Bioengineering 2015, 2, 66–93. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K.; Grewell, D.; Sung, S.; Van Leeuwen, J. Ultrasound applications in wastewater sludge pretreatment: A review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 277–313. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Application of physico-chemical pretreatment methods to enhance the sludge disintegration and subsequent anaerobic digestion: An up to date review. Rev. Environ. Sci. Bio/Technol. 2011, 10, 215. [Google Scholar] [CrossRef]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal pretreatment of sewage sludge to enhance anaerobic digestion: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010. [Google Scholar] [CrossRef] [PubMed]
- Kepp, U.; Machenbach, I.; Weisz, N.; Solheim, O.E. Enhanced stabilisation of sewage sludge through thermal hydrolysis-three years of experience with full scale plant. Water Sci. Technol. 2000, 42, 89–96. [Google Scholar] [CrossRef]
- Martínez, E.J.; Gil, M.V.; Rosas, J.G.; Moreno, R.; Mateos, R.; Morán, A.; Gómez, X. Application of thermal analysis for evaluating the digestion of microwave pre-treated sewage sludge. J. Therm. Anal. Calorim. 2017, 127, 1209–1219. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Fei, X.; Wang, S.; Yuan, H. Enhancement of thermophilic anaerobic digestion of thickened waste activated sludge by combined microwave and alkaline pretreatment. J. Environ. Sci. 2011, 23, 1257–1265. [Google Scholar] [CrossRef]
- García-Cascallana, J.; Borge-Díez, D.; Gómez, X. Enhancing the efficiency of thermal hydrolysis process in wastewater treatment plants by the use of steam accumulation. Int. J. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Valo, A.; Carrere, H.; Delgenes, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Sattar, A.; Arslan, C.; Ji, C.; Sattar, S.; Ali Mari, I.; Rashid, H.; Ilyas, F. Comparing the bio-hydrogen production potential of pretreated rice straw co-digested with seeded sludge using an anaerobic bioreactor under mesophilic thermophilic conditions. Energies 2016, 9, 198. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Liu, W.; Ye, M.; Su, F. Effluent characteristics of advanced treatment for biotreated coking wastewater by electrochemical technology using BDD anodes. Environ. Sci. Pollut. Res. 2015, 22, 6827–6834. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.J.; Rosas, J.G.; Gonzalez, R.; Garcia, D.; Gomez, X. Treatment of vinasse by electrochemical oxidation: Evaluating the performance of boron-doped diamond (BDD)-based and dimensionally stable anodes (DSAs). Int. J. Environ. Sci. Technol. 2018, 15, 1159–1168. [Google Scholar] [CrossRef]
- Curteanu, S.; Godini, K.; Piuleac, C.G.; Azarian, G.; Rahmani, A.R.; Butnariu, C. Electro-oxidation method applied for activated sludge treatment: Experiment and simulation based on supervised machine learning methods. Ind. Eng. Chem. Res. 2014, 53, 4902–4912. [Google Scholar] [CrossRef]
- Rizzardini, C.B.; Goi, D. Sustainability of domestic sewage sludge disposal. Sustainability 2014, 6, 2424–2434. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Zhao, Y.; Zhu, X. New sludge pretreatment method to improve methane production in waste activated sludge digestion. Environ. Sci. Technol. 2010, 44, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Bougrier, C.; Albasi, C.; Delgenès, J.P.; Carrère, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. Process Intensif. 2006, 45, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Ahn, J.H.; Kim, J.; Hwang, S. Use of microwave pretreatment for enhanced anaerobiosis of secondary sludge. Water Sci. Technol. 2004, 50, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Erden, G.; Filibeli, A. Improving anaerobic biodegradability of biological sludges by Fenton pre-treatment: Effects on single stage and two-stage anaerobic digestion. Desalination 2010, 251, 58–63. [Google Scholar] [CrossRef]
- Feki, E.; Khoufi, S.; Loukil, S.; Sayadi, S. Improvement of anaerobic digestion of waste-activated sludge by using H2O2 oxidation, electrolysis, electro-oxidation and thermo-alkaline pretreatments. Environ. Sci. Pollut. Res. 2015, 22, 14717–14726. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Xu, J.; Yuan, H.; Lou, Z.; Lin, J.; Zhu, N. Enhancement of anaerobic digestion of waste activated sludge by electrochemical pretreatment. Fuel 2014, 130, 279–285. [Google Scholar] [CrossRef]
- Kavitha, S.; Pray, S.S.; Yogalakshmi, K.N.; Kumar, S.A.; Yeom, I.T. Effect of chemo-mechanical disintegration on sludge anaerobic digestion for enhanced biogas production. Environ. Sci. Pollut. Res. 2016, 23, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Jayashree, C.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour. Technol. 2014, 168, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.J.; Meng, S.J.; Kiran, E.U.; Liu, Y. Enzymatic pretreatment of activated sludge, food waste and their mixture for enhanced bioenergy recovery and waste volume reduction via anaerobic digestion. Appl. Energy 2016, 179, 1131–1137. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Bidini, G.; Fantozzi, F. Codigestion of untreated and treated sewage sludge with the organic fraction of municipal solid wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-Digestion of Napier Grass and Its Silage with Cow Dung for Methane Production. Energies 2017, 10, 1654. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X. Biogas production from anaerobic co-digestion of food waste with dairy manure in a two-phase digestion system. Appl. Biochem. Biotechnol. 2010, 160, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Marañón, E.; Castrillón, L.; Quiroga, G.; Fernández-Nava, Y.; Gómez, L.; García, M.M. Co-digestion of cattle manure with food waste and sludge to increase biogas production. Waste Manag. 2012, 32, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Blanco, D.; Fierro, J.; Martínez, E.J.; Gómez, X. Anaerobic co-digestion of sewage sludge with cheese whey under thermophilic and mesophilic conditions. Int. J. Energy Eng. 2014, 4, 26–31. [Google Scholar] [CrossRef]
- González, R.; Smith, R.; Blanco, D.; Fierro, J.; Gómez, X. Application of thermal analysis for evaluating the effect of glycerine addition on the digestion of swine manure. J. Therm. Anal. Calorim. 2018. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci. Technol. 2014, 69, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.P.; Mandale, S.J.; Williams, P.M.; Lovitt, R.W. Nanofiltration of treated digested agricultural wastewater for recovery of carboxylic acids. J. Clean. Prod. 2016, 112, 4749–4761. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valoriz. 2013, 4, 557–581. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Piveteau, S.; Picard, S.; Dabert, P.; Daumer, M.L. Dissolution of particulate phosphorus in pig slurry through biological acidification: A critical step for maximum phosphorus recovery as struvite. Water Res. 2017, 124, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Gomez, X.; Escapa, A.; Moran, A. Evaluation and simultaneous optimization of bio-hydrogen production using 32 factorial design and the desirability function. J. Power Sources 2007, 169, 131–139. [Google Scholar] [CrossRef]
- Akinbomi, J.; Wikandari, R.; Taherzadeh, M.J. Enhanced fermentative hydrogen and methane production from an inhibitory fruit-flavored medium with membrane-encapsulated cells. Membranes 2015, 5, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A.; Shipley, G. Utilising biohydrogen to increase methane production, energy yields and process efficiency via two stage anaerobic digestion of grass. Bioresour. Technol. 2015, 189, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, W.; Yang, C.; Wang, L.; Liang, B.; Thangavel, S.; Guo, Z.; Wang, A. Biocathodic methanogenic community in an integrated anaerobic digestion and microbial electrolysis system for enhancement of methane production from waste sludge. ACS Sustain. Chem. Eng. 2016, 4, 4913–4921. [Google Scholar] [CrossRef]
- Liu, W.; He, Z.; Yang, C.; Zhou, A.; Guo, Z.; Liang, B.; Varrone, C.; Wang, A.J. Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol. Biofuels 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: Process performance and microbial insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Fernández, C.; Gómez, X.; Morán, A. Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol. Bioprocess Eng. 2011. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Teater, C.; Liu, Y.; Maclellan, J.; Liao, W. A sustainable pathway of cellulosic ethanol production integrating anaerobic digestion with biorefining. Biotechnol. Bioeng. 2010, 105, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Smith, M.C.; Kondrad, S.L.; White, J.W. Evaluation of biogas production potential by dry anaerobic digestion of switchgrass–animal manure mixtures. Appl. Biochem. Biotechnol. 2010, 160, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, Z.; Hu, Y.; Wang, X.; Wang, G.; Zuo, J.; Wang, K.; Qian, Y. Microbial Insight into a Pilot-Scale Enhanced Two-Stage High-Solid Anaerobic Digestion System Treating Waste Activated Sludge. Int. J. Environ. Res. Public Health 2017, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Fierro, J.; Martínez, J.E.; Rosas, J.G.; Blanco, D.; Gómez, X. Anaerobic codigestion of poultry manure and sewage sludge under solid-phase configuration. Environ. Prog. Sustain. 2014, 33, 866–872. [Google Scholar] [CrossRef]
- Smeller, L. Pressure-temperature phase diagrams of biomolecules. Biochim. Biophys. Acta 2002, 1595, e11–e29. [Google Scholar] [CrossRef]
- Mayumi, D.; Mochimaru, H.; Yoshioka, H.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Takeuchi, M.; Kamagata, Y. Evidence for syntrophic acetate oxidation coupled to hydrogenotrophic methanogenesis in the high-temperature petroleum reservoir of Yabase oil field (Japan). Environ. Microbiol. 2011, 13, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Birrien, J.-L.; Fouquet, Y.; Cherkashov, G.; Jebbar, M.; Querellou, J.; Oger, P.; Cambon-Bonavita, M.-A.; Xiao, X.; Prieur, D. Pyrococcus CH1, an obligate piezophilic hyperthermophile: Extending the upper pressure-temperature limits for life. ISME J. 2009, 3, e873–e876. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Miyazaki, M.; Hirayama, H.; Nakagawa, S.; Querellou, J.; Godfroy, A. Isolation and physiological characterization of two novel, piezophilic, thermophilic chemolithoautotrophs from a deep-sea hydrothermal vent chimney. Environ. Microbiol. 2009, 11, e1983–e1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oger, P.M.; Jebbar, M. The many ways of coping with pressure. Res. Microbiol. 2010, 161, 799–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, F.; Horikoshi, K. The biotechnological potential of piezophiles. Trends Biotechnol. 2001, 19, 102–108. [Google Scholar] [CrossRef]
- Mayumi, D.; Dolfing, J.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Tamaki, H.; Takeuchi, M.; Nakatsu, C.H.; Kamagata, Y. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 2013, 4, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Rößler, B.; Zielonka, S.; Wonneberger, A.M.; Lemmer, A. Effects of organic loading rate on the performance of a pressurized anaerobic filter in two-phase anaerobic digestion. Energies 2014, 7, 736–750. [Google Scholar] [CrossRef]
- Lindeboom, R.E.F.; Fermoso, F.G.; Weijma, J.; Zagt, K.; Van Lier, J.B. Autogenerative high pressure digestion: Anaerobic digestion and biogas upgrading in a single step reactor system. Water Sci. Technol. 2011, 64, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Bond, D.R.; Lovley, D.R. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Tender, L.M.; Reimers, C.E.; Stecher, H.A., III; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 2002, 20, 821. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Hoban, D.J.; Van Den Berg, L. Effect of iron on conversion of acetic acid to methane during methanogenic fermentations. J. Appl. Bacteriol. 1979, 47, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Yu, Q.; Xu, Z.; Quan, X. Enhanced high-solids anaerobic digestion of waste activated sludge by the addition of scrap iron. Bioresour. Technol. 2014, 159, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Lovley, D.R. Lack of production of electron-shuttling compounds or solubilization of Fe (III) during reduction of insoluble Fe (III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 2000, 66, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species. Int. J. Mol. Sci. 2017, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xu, S.; Jin, Y.; Han, R.; Liu, H.; Lü, F. Evaluation of methanogenic microbial electrolysis cells under closed/open circuit operations. Environ. Technol. 2018, 39, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Madamwar, D. Anaerobic digestion of a mixture of cheese whey, poultry waste and cattle dung: A study of the use of adsorbents to improve digester performance. Environ. Pollut. 1994, 86, 337–340. [Google Scholar] [CrossRef]
- Patel, V.; Patel, A.; Datta, M. Effects of adsorbents on anaerobic digestion of water hyacinth-cattle dung. Bioresour. Technol. 1992, 40, 179–181. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Borja, R.; Sánchez, E.; Duran, M.M. Effect of the clay mineral zeolite on ammonia inhibition of anaerobic thermophilic reactors treating cattle manure. J. Environ. Sci. Health Part A 1996, 31, 479–500. [Google Scholar] [CrossRef]
- Lin, L.; Wan, C.; Liu, X.; Lei, Z.; Lee, D.J.; Zhang, Y.; Tay, J.H.; Zhang, Z. Anaerobic digestion of swine manure under natural zeolite addition: VFA evolution, cation variation, and related microbial diversity. Appl. Microbiol. Biotechnol. 2013, 97, 10575–10583. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Belostotskiy, D.E.; Ilinskaya, O.N.; Boulygina, E.A.; Grigoryeva, T.V.; Ziganshin, A.M. Effect of the organic loading rate increase and the presence of zeolite on microbial community composition and process stability during anaerobic digestion of chicken wastes. Microb. Ecol. 2015, 70, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Petersen, S.P.; Ahring, B.K. Effects of lipids on thermophilic anaerobic digestion and reduction of lipid inhibition upon addition of bentonite. Appl. Microbiol. Biotechnol. 1990, 33, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Improving thermophilic anaerobic digestion of swine manure. Water Res. 1999, 33, 1805–1810. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Morán, A.; Otero, M.; Gómez, X. Anaerobic co-digestion of poultry blood with OFMSW: FTIR and TG–DTG study of process stabilization. Environ. Technol. 2009, 30, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Bernet, N.; Delgenès, J.P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Martínez, E.J.; Escapa, A.; Martínez, O.; Díez-Antolínez, R.; Gómez, X. Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation 2018, 4, 2. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Cai, W.; Guo, Z.; Wang, L.; Yang, C.; Varrone, C.; Wang, A. Microbial electrolysis contribution to anaerobic digestion of waste activated sludge, leading to accelerated methane production. Renew. Energy 2016, 91, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron-graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.M.K.; Mahammadunnisa, S.K.; Ramaraju, B.; Sreedhar, B.; Subrahmanyam, C. Low-cost adsorbents from bio-waste for the removal of dyes from aqueous solution. Environ. Sci. Pollut. Res. 2013, 20, 4111–4124. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Contescu, C.; Adhikari, S.; Gallego, N.; Evans, N.; Biss, B. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. Carbon 2018, 4, 51. [Google Scholar] [CrossRef]
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Xie, Y.; Ma, Q.; Wu, L. The Short-Term Effects of Rice Straw Biochar, Nitrogen and Phosphorus Fertilizer on Rice Yield and Soil Properties in a Cold Waterlogged Paddy Field. Sustainability 2018, 10, 537. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Gale, N.V.; Dranga, S.; Hudson, V.; Liu, N.; Michener, J.; Thurston, E.; Williams, C.; Smith, S.M.; Thomas, S.C. Is There a Positive Synergistic Effect of Biochar and Compost Soil Amendments on Plant Growth and Physiological Performance? Agronomy 2017, 7, 13. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Paneque, M.; Hilber, I.; Blum, F.; Knicker, H.E.; Bucheli, T.D. Assessment of polycyclic aromatic hydrocarbons in biochar and biochar-amended agricultural soil from Southern Spain. J. Soils Sediments 2016, 16, 557–565. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag. Res. 2016, 34, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Luo, C.; Shao, L.; He, P. Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Res. 2016, 90, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Meyer-Kohlstock, D.; Haupt, T.; Heldt, E.; Heldt, N.; Kraft, E. Biochar as additive in biogas-production from bio-waste. Energies 2016, 9, 247. [Google Scholar] [CrossRef]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Viggi, C.C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S.; Navarra, M.A.; Pettiti, I.; Koch, C.; et al. Enhancing methane production from food waste fermentate using biochar: The added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnol. Biofuels 2017. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Hübner, T.; Mumme, J. Integration of pyrolysis and anaerobic digestion-use of aqueous liquor from digestate pyrolysis for biogas production. Bioresour. Technol. 2015, 183, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Salman, C.A.; Schwede, S.; Thorin, E.; Yan, J. Predictive modelling and simulation of integrated pyrolysis and anaerobic digestion process. Enrgy Procedia 2017, 105, 850–857. [Google Scholar] [CrossRef]



| Pre-Treatment | Biogas Yield | Improvement (%) | Reference |
|---|---|---|---|
| Ultrasound | 261 mL CH4/g VS | 30 | [24] |
| Thermal (170 °C) | 333 mL CH4/g COD | 50 | [41] |
| Ozonation | 259 mL CH4/g COD | 17 | [41] |
| Microwave | 183 mL biogas/Lr 1 d (HRT 2 = 10 d) | 37 | [42] |
| Fenton | 547 mL CH4/g VS | 37 | [43] |
| Electro-oxidation (Fe electrode–H2O2) | 147 mL biogas/g VS | 256 | [44] |
| Electrochemical (Ti/RuO2) | 647 mL biogas/g VS | 63.4 | [45] |
| Surfactant addition and mechanical disintegration | 180 mL CH4/g VS | 260 | [46] |
| Source of Biochar | Substrate | Main Results | Reference |
|---|---|---|---|
| Fruitwood | Glucose (4–8 g/L) | Biochar shortened the methanogenic lag phase and increased maximum methane production rate in 86.6% at 4 g/L of glucose concentration and just 5.2% at the highest glucose concentration (8 g/L) | [121] |
| Fruitwood | Food wastes (4–10 g Dry Weight/L) | Biochar shortened the methanogenic lag phase and increased maximum methane production rate by 123% | [122] |
| Almond shell | Swine manure | Increased in methane yield of 39%. Addition of biochar enhanced the degradation of proteins | [123] |
| Fruitwood | Glucose N: 0.26–7 g/L | Biochar accelerated the initiation of mechanisation during anaerobic digestion under double inhibition risk from both ammonium and acids. Increased maximum methane production rate by 38% | [124] |
| Corn Stover | Sewage sludge | Biochar-amended digesters produced near pipeline-quality biomethane (>90% CH4 and <5 ppb H2S). The biochar addition also increased alkalinity and mitigated ammonia inhibition. Increased maximum methane production rate by 27.6% | [125] |
| Clean forestry wood residue (Holm Oak) | Organic fraction of municipal solid waste (solid state fermentation) | Methane yield increased around 5% with biochar addition | [126] |
| Vineyard pruning | Orange peels | Increased in methane yield of 56%. Biochar addition favoured the electro-active microorganisms consortia creating a synthrophic metabolism between eubacterial and archaeal populations | [127] |
| Wheat bran pellets, coppiced woodlands, and orchard pruning | Food waste fermentate from acidogenic reactor | Conversion of VFAs proceeded at a rate up to 5 times higher than that observed in the unamended controls. Biochar affected the composition of the microbial consortium. Positive effect observed was directly related to the electron-donating capacity (EDC) of the material, but was independent of its bulk electrical conductivity and specific surface area | [128] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, J.; Sánchez, M.E.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C 2018, 4, 59. https://0-doi-org.brum.beds.ac.uk/10.3390/c4040059
González J, Sánchez ME, Gómez X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C. 2018; 4(4):59. https://0-doi-org.brum.beds.ac.uk/10.3390/c4040059
Chicago/Turabian StyleGonzález, Judith, Marta E. Sánchez, and Xiomar Gómez. 2018. "Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials" C 4, no. 4: 59. https://0-doi-org.brum.beds.ac.uk/10.3390/c4040059