A Rational Investigation of the Lewis Acid-Promoted Coupling of Carbon Dioxide with Cyclohexene Oxide: Towards CO2-Sourced Polycyclohexene Carbonate under Solvent- and Cocatalyst-Free Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR)
2.3. High-Pressure Transmission Infrared Spectroscopy
2.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.5. Steric Extrusion Chromatography (SEC)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Coupling of Cyclohexene Oxide with CO2: Catalysts Screening
3. Results and Discussion
3.1. Catalytic Studies Performed with Metal Triflates Complexes
3.1.1. Influence of the Nature of the Metal
3.1.2. Influence of the Nature of the Cocatalyst
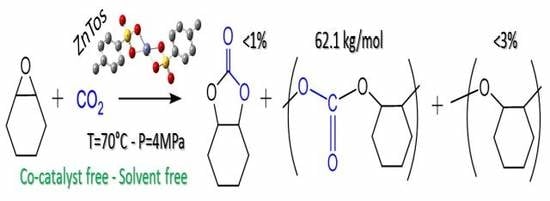
3.2. Catalytic Studies Performed with Metal Tosylates Complexes
3.3. Infrared Absorption Experiments
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Coates, G.W.; Moore, D.R. Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J. Making plastics from carbon dioxide: Salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Macdowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef] [PubMed]
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Tech. 2012, 2, 2169–2187. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Lang, X.-D.; He, X.; Li, Z.-M.; He, L.-N. New routes for CO2 activation and subsequent conversion. Curr. Opin. Green Sustain. Chem. 2017, 7, 31–38. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’elia, V.; Kühn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalton Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef]
- Rintjema, J.; Kleij, A.W. Substrate-assisted carbon dioxide activation as a versatile approach for heterocyclic synthesis. Synthesis 2016, 48, 3863–3878. [Google Scholar]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Tech. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Gennen, S.; Mereau, R.; Detrembleur, C.; Jerome, C.; Tassaing, T. Organocatalytic promoted coupling of carbon dioxide with epoxides: A rational investigation of the cocatalytic activity of various hydrogen bond donors. Catal. Sci. Tech. 2015, 5, 4636–4643. [Google Scholar] [CrossRef]
- Lu, X.B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef] [PubMed]
- Taherimehr, M.; Pescarmona, P.P. Green polycarbonates prepared by the copolymerization of CO2 with epoxides. J. Appl. Polym. Sci. 2014, 131, 41141. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Comm. 2015, 51, 6459–6479. [Google Scholar] [CrossRef]
- Taherimehr, M.; Al-Amsyar, S.M.; Whiteoak, C.J.; Kleij, A.W.; Pescarmona, P.P. High activity and switchable selectivity in the synthesis of cyclic and polymeric cyclohexene carbonates with iron amino triphenolate catalysts. Green Chem. 2013, 15, 3083–3090. [Google Scholar] [CrossRef]
- Ang, R.-R.; Sin, L.T.; Bee, S.-T.; Tee, T.-T.; Kadhum, A.a.H.; Rahmat, A.R.; Wasmi, B.A. Determination of zinc glutarate complexes synthesis factors affecting production of propylene carbonate from carbon dioxide and propylene oxide. Chem. Eng. J. 2017, 327, 120–127. [Google Scholar] [CrossRef]
- Wulf, C.; Doering, U.; Werner, T. Copolymerization of CO2 and epoxides mediated by zinc organyls. RSC Adv. 2018, 8, 3673–3679. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; He, C.-T.; Qin, J.; Li, Z.; Wang, S.; Xiao, M.; Meng, Y. Kinetic and mechanistic investigation for the copolymerization of CO2 and cyclohexene oxide catalyzed by trizinc complexes. Polym. Chem. 2017, 8, 3632–3640. [Google Scholar] [CrossRef]
- Reiter, M.; Vagin, S.; Kronast, A.; Jandl, C.; Rieger, B. A Lewis acid [small beta]-diiminato-zinc-complex as all-rounder for co- and terpolymerisation of various epoxides with carbon dioxide. Chem. Sci. 2017, 8, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Marbach, J.; Nornberg, B.; Rahlf, A.F.; Luinstra, G.A. Zinc glutarate-mediated copolymerization of CO2 and PO - parameter studies using design of experiments. Catal. Sci. Tech. 2017, 7, 2897–2905. [Google Scholar] [CrossRef]
- Sudakar, P.; Sivanesan, D.; Yoon, S. Copolymerization of Epichlorohydrin and CO2 Using Zinc Glutarate: An Additional Application of ZnGA in Polycarbonate Synthesis. Macromol. Rapid Commun. 2016, 37, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xu, B.; Zhang, Y.; Yuan, D.; Yao, Y. Cooperative rare earth metal-zinc based heterometallic catalysts for copolymerization of CO2 and cyclohexene oxide. Green Chem. 2016, 18, 4270–4275. [Google Scholar] [CrossRef]
- Martinez, J.; Castro-Osma, J.A.; Lara-Sanchez, A.; Otero, A.; Fernandez-Baeza, J.; Tejeda, J.; Sanchez-Barba, L.F.; Rodriguez-Dieguez, A. Ring-opening copolymerisation of cyclohexene oxide and carbon dioxide catalysed by scorpionate zinc complexes. Polym. Chem. 2016, 7, 6475–6484. [Google Scholar] [CrossRef]
- Kissling, S.; Lehenmeier, M.W.; Altenbuchner, P.T.; Kronast, A.; Reiter, M.; Deglmann, P.; Seemann, U.B.; Rieger, B. Dinuclear zinc catalysts with unprecedented activities for the copolymerization of cyclohexene oxide and CO2. Chem. Comm. 2015, 51, 4579–4582. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiao, M.; Wang, S.; Pan, M.; Meng, Y. Activities comparison of Schiff base zinc and tri-zinc complexes for alternating copolymerization of CO2 and epoxides. Polym. Chem. 2014, 5, 3838–3846. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, M.; Xu, Y.; Wang, S.; Meng, Y. Zinc adipate/tertiary amine catalytic system: Efficient synthesis of high molecular weight poly(propylene carbonate). J. Polym. Res. 2013, 20, 190. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Z.; Cheng, R.; Yang, Y.; Zhong, L.; He, X.; Liu, B. Mechanism for alternating copolymerization of CO2 and propylene oxide in diethylzinc–water catalytic system: A DFT study. J. CO2 Util. 2013, 2, 39–48. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Herdtweck, E.; Deglmann, P.; Ott, A.K.; Rieger, B. Mechanistic Insights into Heterogeneous Zinc Dicarboxylates and Theoretical Considerations for CO2–Epoxide Copolymerization. J. Am. Chem. Soc. 2011, 133, 13151–13161. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Rainey, P.; Yarbrough, J.C. Bis-salicylaldiminato complexes of zinc. Examination of the catalyzed epoxide/CO2 copolymerization. Inorg. Chem. 2001, 40, 986–993. [Google Scholar] [CrossRef]
- Cheng, M.; Moore, D.R.; Reczek, J.J.; Chamberlain, B.M.; Lobkovsky, E.B.; Coates, G.W. Single-site β-diiminate zinc catalysts for the alternating copolymerization of CO2 and epoxides: Catalyst synthesis and unprecedented polymerization activity. J. Am. Chem. Soc. 2001, 123, 8738–8749. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kwon, H.Y.; Lee, S.Y.; Na, S.J.; Han, S.-I.; Yun, H.; Lee, H.; Park, Y.-W. Bimetallic Anilido-Aldimine Zinc Complexes for Epoxide/CO2 Copolymerization. J. Am. Chem. Soc. 2005, 127, 3031–3037. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, H.; Ullah, A. Rapid copolymerization of canola oil derived epoxide monomers with anhydrides and carbon dioxide (CO2). Polym. Chem. 2017, 8, 6431–6442. [Google Scholar] [CrossRef]
- Deacy, A.C.; Durr, C.B.; Garden, J.A.; White, A.J.P.; Williams, C.K. Groups 1, 2 and Zn(II) Heterodinuclear Catalysts for Epoxide/CO2 Ring-Opening Copolymerization. Inorg. Chem. 2018, 57, 15575–15583. [Google Scholar] [CrossRef] [PubMed]
- Ikpo, N.; Flogeras, J.C.; Kerton, F.M. Aluminium coordination complexes in copolymerization reactions of carbon dioxide and epoxides. Dalton Trans. 2013, 42, 8998–9006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, X.; Wu, W.; Qin, Y.; Wang, X.; Wang, F. Efficient synthesis and stabilization of poly(propylene carbonate) from delicately designed bifunctional aluminum porphyrin complexes. Polym. Chem. 2015, 6, 4719–4724. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yarbrough, J.C.; Ortiz, C.; Fang, C.C. Comparative kinetic studies of the copolymerization of cyclohexene oxide and propylene oxide with carbon dioxide in the presence of chromium salen derivatives. in situ ftir measurements of copolymer vs cyclic carbonate production. J. Am. Chem. Soc. 2003, 125, 7586–7591. [Google Scholar] [CrossRef]
- Devaine-Pressing, K.; Dawe, L.N.; Kozak, C.M. Cyclohexene oxide/carbon dioxide copolymerization by chromium(iii) amino-bis(phenolato) complexes and MALDI-TOF MS analysis of the polycarbonates. Polym. Chem. 2015, 6, 6305–6315. [Google Scholar] [CrossRef] [Green Version]
- Devaine-Pressing, K.; Kozak, C.M. Mechanistic Studies of Cyclohexene Oxide/CO2 Copolymerization by a Chromium(III) Pyridylamine-Bis(Phenolate) Complex. ChemSuschem 2017, 10, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Ulusoy, M.; Karroonnirum, O.; Poland, R.R.; Reibenspies, J.H.; Çetinkaya, B. Highly selective and reactive (salan)crcl catalyst for the copolymerization and block copolymerization of epoxides with carbon dioxide. Macromolecules 2009, 42, 6992–6998. [Google Scholar] [CrossRef]
- Sujith, S.; Min, J.K.; Seong, J.E.; Na, S.J.; Lee, B.Y. A Highly Active and Recyclable Catalytic System for CO2/Propylene Oxide Copolymerization. Angew. Chem. Int. Ed. 2008, 47, 7306–7309. [Google Scholar]
- Li, C.-H.; Chuang, H.-J.; Li, C.-Y.; Ko, B.-T.; Lin, C.-H. Bimetallic nickel and cobalt complexes as high-performance catalysts for copolymerization of carbon dioxide with cyclohexene oxide. Polym. Chem. 2014, 5, 4875–4878. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Sun, X.Y. Copolymerization of Epoxides and CO2 by Cobalt(II) oxaporphyrins with mechanistic explorations on poly(propylene carbonate) formation. Macromol. Chem. Phys. 2018, 219, 1700478. [Google Scholar] [CrossRef]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistic aspects of the copolymerization of CO2 with epoxides using a thermally stable single-site cobalt(III) catalyst. J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef] [PubMed]
- Kember, M.R.; Williams, C.K. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; using water to synthesize polycarbonate polyols. J Am Chem Soc. 2012, 134, 15676–15679. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Ding, K. intramolecularly dinuclear magnesium complex catalyzed copolymerization of cyclohexene oxide with CO2 under ambient CO2 pressure: Kinetics and mechanism. Macromolecules 2006, 39, 128–137. [Google Scholar] [CrossRef]
- Taherimehr, M.; Sertã, J.P.C.C.; Kleij, A.W.; Whiteoak, C.J.; Pescarmona, P.P. New iron pyridylamino-bis(phenolate) catalyst for converting CO2 into cyclic carbonates and cross-linked polycarbonates. ChemSuschem 2015, 8, 1034–1042. [Google Scholar] [CrossRef]
- Buchard, A.; Kember, M.R.; Sandeman, K.G.; Williams, C.K. A bimetallic iron(III) catalyst for CO2/epoxide coupling. Chem. Comm. 2011, 47, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Maity, B.; Pehl, T.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Rieger, B.; Cavallo, L.; Capacchione, C. [OSSO]-type iron(III) complexes for the low-pressure reaction of carbon dioxide with epoxides: Catalytic activity, reaction kinetics, and computational study. ACS Catal. 2018, 8, 6882–6893. [Google Scholar] [CrossRef]
- Nakano, K.; Kobayashi, K.; Ohkawara, T.; Imoto, H.; Nozaki, K. Copolymerization of epoxides with carbon dioxide catalyzed by iron–corrole complexes: Synthesis of a crystalline copolymer. J. Am. Chem. Soc. 2013, 135, 8456–8459. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Buonerba, A.; Capacchione, C. Homogeneous iron catalysts in the reaction of epoxides with carbon dioxide. Adv. Synth. Catal. 2019, 361, 265–282. [Google Scholar] [CrossRef]
- Quadri, C.C.; Le Roux, E. Copolymerization of cyclohexene oxide with CO2 catalyzed by tridentate N-heterocyclic carbene titanium(IV) complexes. Dalton Trans. 2014, 43, 4242–4246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Coupling reaction between CO2 and cyclohexene oxide: Selective control from cyclic carbonate to polycarbonate by ligand design of salen/salalen titanium complexes. Catal. Sci. Tech. 2014, 4, 3964–3972. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Huang, B.-H.; Hsiao, M.-W.; Lin, C.-C.; Ko, B.-T. Structurally diverse copper complexes bearing nno-tridentate schiff-base derivatives as efficient catalysts for copolymerization of carbon dioxide and cyclohexene oxide. Inorg. Chem. 2014, 53, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Decortes, A.; Haak, R.M.; Martín, C.; Belmonte, M.M.; Martin, E.; Benet-Buchholz, J.; Kleij, A.W. Copolymerization of CO2 and cyclohexene oxide mediated by yb(salen)-based complexes. Macromolecules 2015, 48, 8197–8207. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A 2016, 374. [Google Scholar] [CrossRef]
- Ang, R.-R.; Tin Sin, L.; Bee, S.-T.; Tee, T.-T.; Kadhum, A.a.H.; Rahmat, A.R.; Wasmi, B.A. A review of copolymerization of green house gas carbon dioxide and oxiranes to produce polycarbonate. J. Cleaner Prod. 2015, 102, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Sheng, X.; Liu, S.; Ren, G.; Wang, X.; Wang, F. Recent advances in carbon dioxide based copolymers. J. CO2 Util. 2015, 11, 3–9. [Google Scholar] [CrossRef]
- Chapman, A.M.; Keyworth, C.; Kember, M.R.; Lennox, A.J.J.; Williams, C.K. Adding value to power station captured CO2: Tolerant zn and mg homogeneous catalysts for polycarbonate polyol production. ACS Catal. 2015, 5, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, D.; Boopathi, S.K.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Metal-free alternating copolymerization of CO2 with epoxides: Fulfilling “green” synthesis and activity. J. Am. Chem. Soc. 2016, 138, 11117–11120. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 2015, 17, 300–306. [Google Scholar] [CrossRef]
- Shaarani, F.W.; Bou, J.J. Synthesis of vegetable-oil based polymer by terpolymerization of epoxidized soybean oil, propylene oxide and carbon dioxide. Sci. Total Environ. 2017, 598, 931–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stößer, T.; Li, C.; Unruangsri, J.; Saini, P.K.; Sablong, R.J.; Meier, M.A.R.; Williams, C.K.; Koning, C. Bio-derived polymers for coating applications: Comparing poly(limonene carbonate) and poly(cyclohexadiene carbonate). Polym. Chem. 2017, 8, 6099–6105. [Google Scholar]
- Holmes, S.M.; Mckinley, S.G.; Girolami, G.S. Transition metal p-toluenesulfonates. Inorg. Synth. 2002, 33, 91–103. [Google Scholar]
- Bergeot, V.; Tassaing, T.; Besnard, M.; Cansell, F.; Mingotaud, A.-F. Anionic ring-opening polymerization of ε-caprolactone in supercritical carbon dioxide: Parameters influencing the reactivity. J. Supercrit. Fluids 2004, 28, 249–261. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Rodgers, J.L.; Mackiewicz, R.M.; Phelps, A.L. Probing the mechanistic aspects of the chromium salen catalyzed carbon dioxide/epoxide copolymerization process using in situ ATR/FTIR. Catal. Today 2004, 98, 485–492. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific intermolecular interaction of carbon dioxide with polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Jutz, F.; Baiker, A. Supercritical CO2/Ionic Liquid Systems: What Can We Extract from Infrared and Raman Spectra? J. Phys. Chem. B 2009, 113, 10249–10254. [Google Scholar] [CrossRef] [PubMed]





| Entry | Catalyst | Conv b (%) | SCHC c (%) | SPCHC d (%) | SPE e (%) |
|---|---|---|---|---|---|
| 1 | Al(OTf)3 | 99 | 0 | 0 | >99 |
| 2 | Ni(OTf)2 | 99 | 0 | 0 | >99 |
| 3 | Mg(OTf)2 | 89 | 0 | 0 | >99 |
| 4 | Sc(OTf)3 | 85 | 0 | 4 | 96 |
| 5 | Y(OTf)3 | 99 | 0 | 7 | 93 |
| 6 | Zn(OTf)2 | 94 | 0 | 9 | 91 |
| Entry | Cat./cocat. | Cat./cocat Ratio | Conv b (%) | SCHC c (%) | SPCHC d (%) | SPE e (%) |
|---|---|---|---|---|---|---|
| 1 | Zn(OTf)2/PPNCl | 1:1 | 69 | 0 | 10 | 90 |
| 2 | Zn(OTf)2/P(Ph)3 | 1:1 | 79 | 0 | 12 | 88 |
| 3 | “ | 1:2 | 76 | 0 | 15 | 85 |
| 4 | “ | 1:3 | 74 | 0 | 14 | 86 |
| 5 | Zn(OTf)2/P(Cy)3 | 1:2 | 76 | 1 | 20 | 79 |
| 6 | “ | 1:3 | 63 | 6 | 36 | 58 |
| 7 | “ | 1:3 f | 82 | 6 | 54 | 40 |
| 8 | “ | 1:3 g | 90 | 10 | 58 | 32 |
| 9 | “ | 1:3 f,h | 76 | 1 | 39 | 60 |
| 10 | “ | 1:3 f,i | 75 | 3 | 46 | 51 |
| 11 | “ | 1:3 f,j | 77 | 2 | 23 | 75 |
| 12 | Zn(OTf)2/Et4NHSO4 | 1:3 f | 87 | 1 | 11 | 88 |
| 13 | Zn(OTf)2/Et4NSCN | 1:3 f | 50 | 48 | 52 | 0 |
| 14 | Zn(OTf)2/Et4NTos | 1:3 f | 74 | 6 | 83 | 11 |
| 15 | Zn(OTf)2/CTATos | 1:3 f | 73 | 6 | 55 | 39 |
| 16 | Zn(OTf)2/PyrTos | 1:3 f | 48 | 8 | 14 | 78 |
| Entry | Cat./cocat. | Cat.:cocat Ratio | Conv b (%) | SCHC c (%) | SPCHC d (%) | SPE e (%) |
|---|---|---|---|---|---|---|
| 1 | Zn(OTf)2/Et4NTos | 1:1 | 99 | 0 | 9 | 91 |
| 2 | “ | 1:1.5 | 99 | 0 | 26 | 74 |
| 3 | “ | 1:1.75 | 88 | 1 | 62 | 37 |
| 4 | “ | 1:2 | 83 | 0 | 78 | 22 |
| 5 | “ | 1:3 | 74 | 6 | 83 | 11 |
| 6 | “ | 1:4 | 70 | 7 | 86 | 7 |
| 7 | Zn(OTf)2/CTATos | 1:1 | 95 | 2 | 17 | 81 |
| 8 | “ | 1:1.5 | 87 | 1 | 55 | 44 |
| 9 | “ | 1:1.75 | 72 | 4 | 75 | 21 |
| 10 | “ | 1:2 | 71 | 6 | 75 | 19 |
| 11 | “ | 1:3 | 73 | 7 | 54 | 39 |
| 12 | “ | 1:4 | 75 | 8 | 69 | 23 |
| Entry | Catalyst | Conv b (%) | SCHC c (%) | SPCHC d (%) | SPE e (%) |
|---|---|---|---|---|---|
| 1 | AgTos | 24 | 2 | 16 | 82 |
| 2 | FeTos | 91 | 0 | 18 | 82 |
| 3 | ZnTos | 67 | 0 | 97 | 3 |
| 4 | ZnTos f | 72 | 1 | 95 | 4 |
| 5 | ZnTos g | 62 | 2 | 94 | 4 |
| 6 | ZnTos h | 98 | 2 | 66 | 32 |
| 7 | ZnTos i | 65 | 1 | 95 | 4 |
| 8 | ZnTos/Toluene (0.2 mL) | 75 | 3 | 85 | 12 |
| 9 | ZnTos/Toluene (0.4 mL) | 63 | 3 | 82 | 15 |
| 10 | ZnTos/Toluene (0.5 mL) | 35 | 2 | 76 | 22 |
| Table/Entry | Cat./cocat. | Solvent (ml) | Conv b (%) | SPCHC c (%) | Mn (g.mol−1) | PDI d | Tge (°C) |
|---|---|---|---|---|---|---|---|
| 2/7 | Zn(OTf)2/P(Cy)3 | “ | 82 | 54 | 12,100 | 4.1 | |
| 3/4 | Zn(OTf)2/Et4NTos | “ | 83 | 78 | 26,000 | 5.1 | |
| 3/9 | Zn(OTf)2/CTATos | “ | 72 | 75 | 24,800 | 5.3 | |
| 4/3 | ZnTos | “ | 67 | 97 | 62,100 | 4.1 | 124.5 |
| 4/4 | ZnTos f | “ | 72 | 95 | 42,400 | 6.9 | 123.7 |
| 4/5 | ZnTos g | “ | 62 | 94 | 39,200 | 8.1 | 121.2 |
| 4/7 | ZnTos h | “ | 65 | 95 | 33,500 | 9.9 | |
| 4/8 | ZnTos | Toluene/(0.2) | 75 | 85 | 41,400 | 10 | |
| 4/9 | ZnTos | Toluene/(0.4) | 63 | 82 | 69,300 | 6.3 | |
| 4/10 | ZnTos | Toluene/(0.5) | 35 | 76 | 109,300 | 4.5 | 124.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grondin, J.; Aupetit, C.; Tassaing, T. A Rational Investigation of the Lewis Acid-Promoted Coupling of Carbon Dioxide with Cyclohexene Oxide: Towards CO2-Sourced Polycyclohexene Carbonate under Solvent- and Cocatalyst-Free Conditions. C 2019, 5, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030039
Grondin J, Aupetit C, Tassaing T. A Rational Investigation of the Lewis Acid-Promoted Coupling of Carbon Dioxide with Cyclohexene Oxide: Towards CO2-Sourced Polycyclohexene Carbonate under Solvent- and Cocatalyst-Free Conditions. C. 2019; 5(3):39. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030039
Chicago/Turabian StyleGrondin, Joseph, Christian Aupetit, and Thierry Tassaing. 2019. "A Rational Investigation of the Lewis Acid-Promoted Coupling of Carbon Dioxide with Cyclohexene Oxide: Towards CO2-Sourced Polycyclohexene Carbonate under Solvent- and Cocatalyst-Free Conditions" C 5, no. 3: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030039