Preparation of Synthesis Gas from CO2 for Fischer–Tropsch Synthesis—Comparison of Alternative Process Configurations
Abstract
:1. Introduction
2. Technology Overview
2.1. Water Electrolysis
2.2. Synthesis Gas Preparation
2.3. Fischer–Tropsch Synthesis
3. Materials and Methods
3.1. Plant Configurations
- Allothermal from combustion;
- Autothermal from partial oxidation;
- Autothermal from electric resistance.
- once-through design (i.e., placement outside the FT recycle loop);
- recycle design (i.e., placement inside the FT recycle loop).
3.1.1. Designs Based on Allothermal Heating from Combustion (OT-COMB and RC-COMB)
3.1.2. Designs Based on Autothermal Heating from Partial Oxidation (OT-POX and RC-POX)
3.1.3. Designs Based on Autothermal Heating from Electric Resistance (OT-ER and RC-ER)
3.2. Evaluation Metrics and Design Parameters
4. Results and Discussion
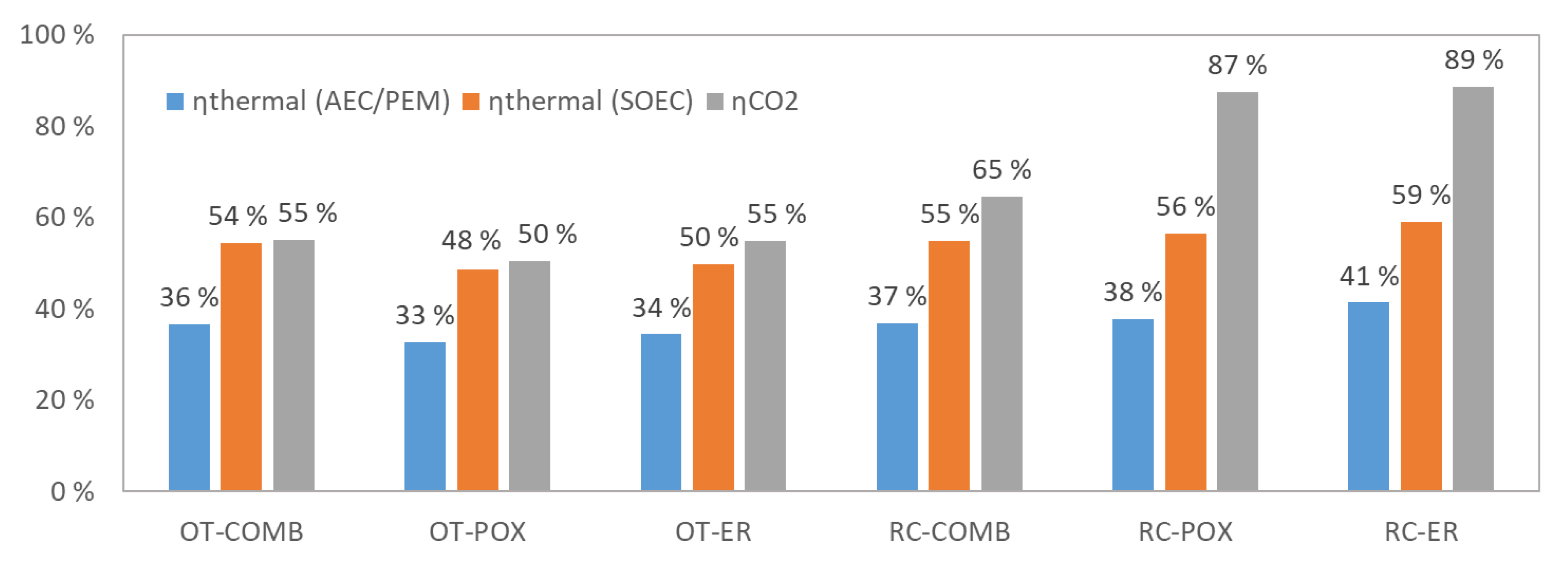
4.1. Mass and Energy Balances
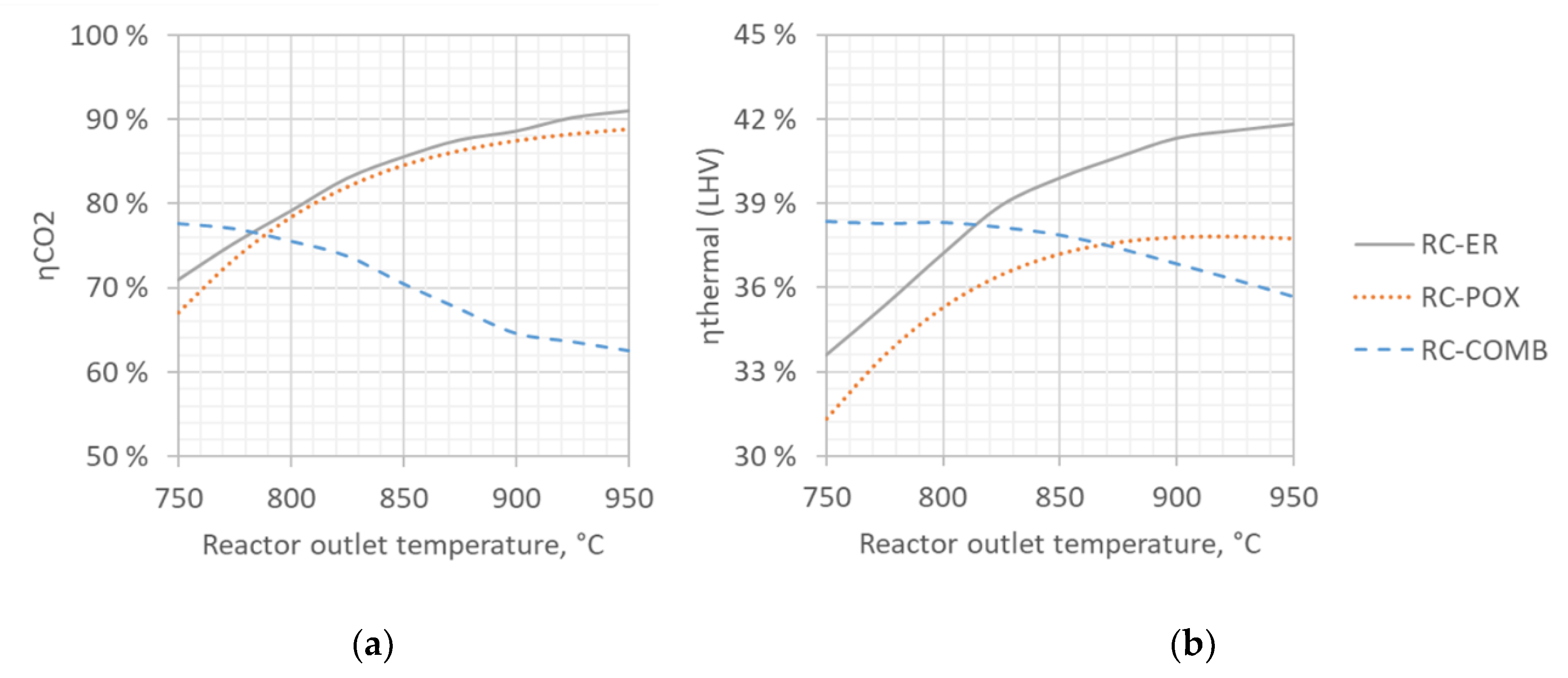
4.2. Sensitivity to Syngas Preparation Temperature
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Stream | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | C | 113 | 25 | 550 | 900 | 220 | 40 | 40 | 40 | 40 |
| Pressure | bar | 20 | 20 | 19.9 | 19.4 | 19.2 | 18.5 | 18.5 | 18.5 | 18.9 |
| Mole Flows | kmol/hr | 100.0 | 229.2 | 329.2 | 317.2 | 707.3 | 140.4 | 3.6 | 437.3 | 47.2 |
| Mole Fractions | ||||||||||
| CO | 0.206 | 0.119 | 0.048 | 0.048 | ||||||
| CO2 | 1.000 | 0.304 | 0.090 | 0.376 | 0.608 | 0.608 | ||||
| H2 | 1.000 | 0.696 | 0.441 | 0.246 | 0.088 | 0.088 | ||||
| N2 | ||||||||||
| CH4 | 0.019 | 0.138 | 0.234 | 0.234 | ||||||
| H2O | 0.000 | 0.244 | 0.109 | 1.000 | ||||||
| O2 | ||||||||||
| C2H6 | 0.004 | 0.008 | 0.008 | |||||||
| C3H8 | 0.004 | 0.007 | 0.007 | |||||||
| C4H10 | 0.004 | 0.007 | 0.007 | |||||||
| C5+ | 1.000 | |||||||||
| Mass Flows | kg/hr | 4401 | 462 | 4863 | 4863 | 17,725 | 2529 | 779 | 14,418 | 1556 |
| (LHV) Net heating value | MJ/kg | 0 | 119.96 | 11.40 | 11.75 | 9.07 | 0 | 43.89 | 8.06 | 8.06 |
| Duty | MW | 0.0 | 15.4 | 15.4 | 15.9 | 44.7 | 0.0 | 9.5 | 32.3 | 3.5 |
| Stream | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | C | 105 | 25 | 152 | 900 | 220 | 40 | 40 | 40 | 40 |
| Pressure | bar | 20 | 20 | 19.9 | 19.4 | 19.2 | 18.5 | 18.5 | 18.5 | 18.5 |
| Mole Flows | kmol/hr | 100.0 | 234.1 | 116.3 | 327.8 | 689.9 | 156.7 | 3.3 | 414.5 | 52.4 |
| Mole Fractions | ||||||||||
| CO | 0.183 | 0.112 | 0.046 | 0.046 | ||||||
| CO2 | 1.000 | 0.860 | 0.112 | 0.421 | 0.701 | 0.701 | ||||
| H2 | 1.000 | 0.393 | 0.233 | 0.088 | 0.088 | |||||
| N2 | ||||||||||
| CH4 | 0.010 | 0.082 | 0.148 | 0.148 | ||||||
| H2O | 0.302 | 0.144 | 1.000 | |||||||
| O2 | 0.140 | |||||||||
| C2H6 | 0.003 | 0.006 | 0.006 | |||||||
| C3H8 | 0.003 | 0.006 | 0.006 | |||||||
| C4H10 | 0.003 | 0.005 | 0.005 | |||||||
| C5+ | 1.000 | |||||||||
| Mass Flows | kg/hr | 4401 | 472 | 4923 | 5395 | 18,226 | 2824 | 713 | 14,690 | 1858 |
| (LHV) Net heating value | MJ/kg | 0 | 119.96 | 0 | 9.39 | 6.52 | 0 | 43.89 | 5.31 | 5.31 |
| Duty | MW | 0.0 | 15.7 | 0.0 | 14.1 | 33.0 | 0.0 | 8.7 | 21.7 | 2.7 |
| Stream | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | C | 105 | 25 | 550 | 900 | 220 | 40 | 40 | 40 | 40 |
| Pressure | bar | 20 | 20 | 19.9 | 19.4 | 19.2 | 18.5 | 18.5 | 18.5 | 18.5 |
| Mole Flows | kmol/hr | 100.0 | 229.5 | 329.5 | 317.6 | 739.2 | 140.6 | 3.6 | 468.5 | 46.9 |
| Mole Fractions | ||||||||||
| CO | 0.206 | 0.114 | 0.045 | 0.045 | ||||||
| CO2 | 1.000 | 0.303 | 0.090 | 0.387 | 0.611 | 0.611 | ||||
| H2 | 1.000 | 0.697 | 0.442 | 0.239 | 0.086 | 0.086 | ||||
| N2 | ||||||||||
| CH4 | 0.019 | 0.143 | 0.236 | 0.236 | ||||||
| H2O | 0.243 | 0.105 | 1.000 | |||||||
| O2 | ||||||||||
| C2H6 | 0.004 | 0.008 | 0.008 | |||||||
| C3H8 | 0.004 | 0.007 | 0.007 | |||||||
| C4H10 | 0.004 | 0.007 | 0.007 | |||||||
| C5+ | 1.000 | |||||||||
| Mass Flows | kg/hr | 4401 | 463 | 4864 | 4864 | 18,802 | 2533 | 782 | 15,487 | 1549 |
| (LHV) Net heating value | MJ/kg | 0 | 119.96 | 11.41 | 11.76 | 9.01 | 0 | 43.89 | 8.05 | 8.05 |
| Duty | MW | 0.0 | 15.4 | 15.4 | 15.9 | 47.1 | 0.0 | 9.5 | 34.6 | 3.5 |
| Stream | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | C | 113 | 25 | 550 | 900 | 220 | 40 | 40 | 40 | 40 |
| Pressure | bar | 20 | 20 | 19.9 | 19.4 | 19.2 | 18.5 | 18.5 | 18.5 | 18.5 |
| Mole Flows | kmol/hr | 100.0 | 270.4 | 441.8 | 440.2 | 440.2 | 160.6 | 4.2 | 127.4 | 55.9 |
| Mole Fractions | ||||||||||
| CO | 0.031 | 0.224 | 0.224 | 0.194 | 0.194 | |||||
| CO2 | 1.000 | 0.268 | 0.074 | 0.074 | 0.256 | 0.256 | ||||
| H2 | 1.000 | 0.674 | 0.474 | 0.474 | 0.386 | 0.386 | ||||
| N2 | ||||||||||
| CH4 | 0.025 | 0.032 | 0.032 | 0.156 | 0.156 | |||||
| H2O | 0.197 | 0.197 | 1.000 | |||||||
| O2 | ||||||||||
| C2H6 | 0.001 | 0.003 | 0.003 | |||||||
| C3H8 | 0.000 | 0.003 | 0.003 | |||||||
| C4H10 | 0.000 | 0.003 | 0.003 | |||||||
| C5+ | 1.000 | |||||||||
| Mass Flows | kg/hr | 4401 | 545 | 6400 | 6400 | 6400 | 2892 | 915 | 2593 | 1139 |
| (LHV) Net heating value | MJ/kg | 0 | 119.96 | 13.47 | 13.98 | 13.98 | 0 | 43.89 | 14.32 | 14.32 |
| Duty | MW | 0.0 | 18.2 | 23.9 | 24.9 | 24.9 | 0.0 | 11.2 | 10.3 | 4.5 |
| Stream | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | C | 105 | 25 | 188 | 900 | 220 | 40 | 40 | 40 | 40 |
| Pressure | bar | 20 | 20 | 19.9 | 19.4 | 19.2 | 18.5 | 18.5 | 18.5 | 18.5 |
| Mole Flows | kmol/hr | 100.0 | 352.5 | 131.7 | 628.3 | 628.3 | 247.1 | 5.6 | 176.1 | 19.9 |
| Mole Fractions | ||||||||||
| CO | 0.212 | 0.212 | 0.189 | 0.189 | ||||||
| CO2 | 1.000 | 0.759 | 0.089 | 0.089 | 0.317 | 0.317 | ||||
| H2 | 1.000 | 0.445 | 0.445 | 0.367 | 0.367 | |||||
| N2 | ||||||||||
| CH4 | 0.021 | 0.021 | 0.119 | 0.119 | ||||||
| H2O | 0.234 | 0.234 | 1.000 | |||||||
| O2 | 0.241 | |||||||||
| C2H6 | 0.003 | 0.003 | ||||||||
| C3H8 | 0.003 | 0.003 | ||||||||
| C4H10 | 0.003 | 0.003 | ||||||||
| C5+ | 1.000 | |||||||||
| Mass Flows | kg/hr | 4401 | 711 | 5415 | 9603 | 9603 | 4451 | 1233 | 3920 | 443 |
| (LHV) Net heating value | MJ/kg | 0 | 119.96 | 0 | 12.03 | 12.03 | 0 | 43.89 | 11.48 | 11.48 |
| Duty | MW | 0.0 | 23.7 | 0.0 | 32.1 | 32.1 | 0.0 | 15.0 | 12.5 | 1.4 |
| Stream | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | C | 105 | 25 | 550 | 900 | 220 | 40 | 40 | 40 | 40 |
| Pressure | bar | 20 | 20 | 19.9 | 19.4 | 19.2 | 18.5 | 18.5 | 18.5 | 18.5 |
| Mole Flows | kmol/hr | 100.0 | 297.0 | 537.7 | 555.4 | 555.4 | 189.1 | 5.7 | 157.4 | 16.7 |
| Mole Fractions | ||||||||||
| CO | 0.056 | 0.244 | 0.244 | 0.215 | 0.215 | |||||
| CO2 | 1.000 | 0.244 | 0.062 | 0.062 | 0.220 | 0.220 | ||||
| H2 | 1.000 | 0.641 | 0.489 | 0.489 | 0.338 | 0.338 | ||||
| N2 | ||||||||||
| CH4 | 0.057 | 0.047 | 0.047 | 0.217 | 0.217 | |||||
| H2O | 0.158 | 0.158 | 1.000 | |||||||
| O2 | ||||||||||
| C2H6 | 0.001 | 0.004 | 0.004 | |||||||
| C3H8 | 0.001 | 0.003 | 0.003 | |||||||
| C4H10 | 0.001 | 0.003 | 0.003 | |||||||
| C5+ | 1.000 | |||||||||
| Mass Flows | kg/hr | 4401 | 599 | 7860 | 7860 | 7860 | 3406 | 1255 | 3198 | 338 |
| (LHV) Net heating value | MJ/kg | 0 | 119.96 | 15.17 | 15.89 | 15.89 | 0 | 43.89 | 16.59 | 16.59 |
| Duty | MW | 0.0 | 19.9 | 33.1 | 34.7 | 34.7 | 0.0 | 15.3 | 14.7 | 1.6 |
References
- Sims, R.; Schaeffer, R.; Creutzig, F.; Cruz-Núñez, X.; D’Agosto, M.; Dimitriu, D.; Meza, M.J.F.; Fulton, L.; Kobayashi, S.; Lah, O.; et al. 2014: Transport. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Hannula, I.; Reiner, D.M. The role of carbon-neutral synthetic fuels and battery electric vehicles in a sustainable transport system. Joule 2019, 3, 2390–2402. [Google Scholar] [CrossRef]
- Tracking Clean Energy Progress—Topics—IEA. Available online: www.iea.org/tcep (accessed on 16 June 2020).
- Mohr, A.; Raman, S. Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Energy Policy 2013, 63, 114–122. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Delivering Sustainable Bioenergy, Technology Roadmap, OECD, Paris; IEA: Paris, France, 2017. [Google Scholar]
- Steinberg, M. Synthetic carbonaceous fuels and feedstocks from oxides of carbon and nuclear power. Fuel 1978, 57, 460–468. [Google Scholar] [CrossRef]
- Zeman, F.S.; Keith, D.W. Carbon neutral hydrocarbons. Philos. Trans. R. Soc. A 2008, 366, 3901–3918. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, I.; Garcia-Gutiérrez, P.; Elder, R.H.; Cuéllar-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Abanades, J.C.; Rubin, E.S.; Mazzotti, M.; Herzog, H.J. On the climate change mitigation potential of CO2 conversion to fuels. Energy Environ. Sci. 2017, 10, 2491–2499. [Google Scholar] [CrossRef]
- Blanco, H.; Faaij, A. A review of the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energ. Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Bruhn, T.; Naims, H.; Olfe-Krautlein, B. Separating the debate on CO2 utilisation from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Wulf, C.; Linßen, J.; Zapp, P. Review of Power-to-Gas Projects in Europe. Energy Procedia 2018, 155, 367–378. [Google Scholar] [CrossRef]
- CRI—Carbon Recycling International. Available online: www.carbonrecycling.is (accessed on 16 June 2020).
- Otten, R. The First Industrial PtG Plant—Audi E-Gas as Driver for the Energy Turnaround. CEDEC Gas Day Verona Italy. 2014. Available online: http://bit.ly/2zTg5kW (accessed on 16 June 2020).
- Soletair—Sustainable Technologies. Available online: https://soletair.fi (accessed on 16 June 2020).
- Vázquez, F.V.; Koponen, J.; Ruuskanen, V.; Bajamundi, C.; Kosonen, A.; Simell, P.; Ahola, J.; Frilund, C.; Elfving, J.; Reinikainen, M.; et al. Power-to-X technology using renewable electricity and carbon dioxide from ambient air: SOLETAIR proof-of-concept and improved process concept. J. CO2 Util. 2018, 28, 235–246. [Google Scholar] [CrossRef]
- Sunfire—Energy Everywhere. Available online: https://www.sunfire.de/en/ (accessed on 16 June 2020).
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Study on Development of Water Electrolysis in the EU; FCH JU: Bruxelles, Belgium, 2014. [Google Scholar]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Kaiser, P.; Unde, R.; Kern, C.; Jess, A. Production of Liquid Hydrocarbons with CO2 as Carbon Source based on Reverse Water—Gas Shift and Fischer—Tropsch Synthesis. Chem. Ing. Tech. 2013, 85, 489–499. [Google Scholar] [CrossRef]
- Hansen, J.B.; Christiansen, N.; Nielsen, J.U. Production of Sustainable Fuels by Means of Solid Oxide Electrolysis. ECS Trans. 2011, 35, 2941–2948. [Google Scholar] [CrossRef]
- Simell, P.; Hannula, I.; Tuomi, S.; Nieminen, M.; Kurkela, E.; Hiltunen, I.; Kaisalo, N.; Kihlman, J. Clean syngas from biomass—Process development and concept assessment. Biomass Convers. Biorefinery 2014, 4, 357–370. [Google Scholar] [CrossRef]
- Sie, S.; Krishna, R. Fundamentals and selection of advanced Fischer-Tropsch reactors. Appl. Catal. A Gen. 1999, 186, 55–70. [Google Scholar] [CrossRef]
- Sie, S.; Senden, M.; Wechem, H.V. Conversion of natural gas to transportation fuels via the shell middle distillate synthesis process (SMDS). Catal. Today 1991, 8, 371–394. [Google Scholar] [CrossRef]
- Anderson, R. Catalysts for the Fischer-Tropsch Synthesis; Van Nostrand-Reinhold: New York, NY, USA, 1956; Volume 4. [Google Scholar]
- De Klerk, A. Fischer-Tropsch fuels refinery design. Energy Environ. Sci. 2011, 4, 1177–1205. [Google Scholar] [CrossRef]
- Eilers, J.; Posthuma, S.; Sie, S. The shell middle distillate synthesis process (smds). Catal. Lett. 1990, 7, 253–269. [Google Scholar] [CrossRef]
- de Klerk, A. Fischer-Tropsch Refining; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- de Klerk, A.; Li, Y.W.; Zennaro, R. Fischer-Tropsch Technology. In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P.M., de Klerk, A., Eds.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Rytter, E.; Tsakoumis, N.E.; Holmen, A. On the selectivity to higher hydrocarbons in Co-based Fischer-Tropsch synthesis. Catal. Today 2016, 261, 3–16. [Google Scholar] [CrossRef]
- LeViness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer–Tropsch Synthesis Technology—New Advances on State-of-the-Art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Moyo, M.; Liu, X.; Hildebrandt, D. A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol. 2019, 192, 105–129. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbæk, J.S.; Vendelbo, S.B.; Bendixen, F.B.; Eriksen, W.L.; Aasberg-Petersen, K.; Frandsen, C.; Chorkendorff, I.; Mortensen, P.M. Electrified methane reforming: A compact approach to greener industrial hydrogen production. Science 2019, 364, 756–759. [Google Scholar] [CrossRef] [Green Version]
- Hannula, I. Co-production of synthetic fuels and district heat from biomass residues, carbon dioxide and electricity: Performance and cost analysis. Biomass Bioenergy 2015, 74, 26–46. [Google Scholar] [CrossRef]
- Hannula, I. Hydrogen enhancement potential of synthetic biofuels manufacture in the European context: A techno-economic assessment. Energy 2016, 104, 199–212. [Google Scholar] [CrossRef]
- Hombach, L.E.; Doré, L.; Heidgen, K.; Maas, H.; Wallington, T.J.; Walther, G. Economic and environmental assessment of current (2015) and future (2030) use of E-fuels in light-duty vehicles in Germany. J. Clean. Prod. 2019, 207, 153–162. [Google Scholar] [CrossRef]


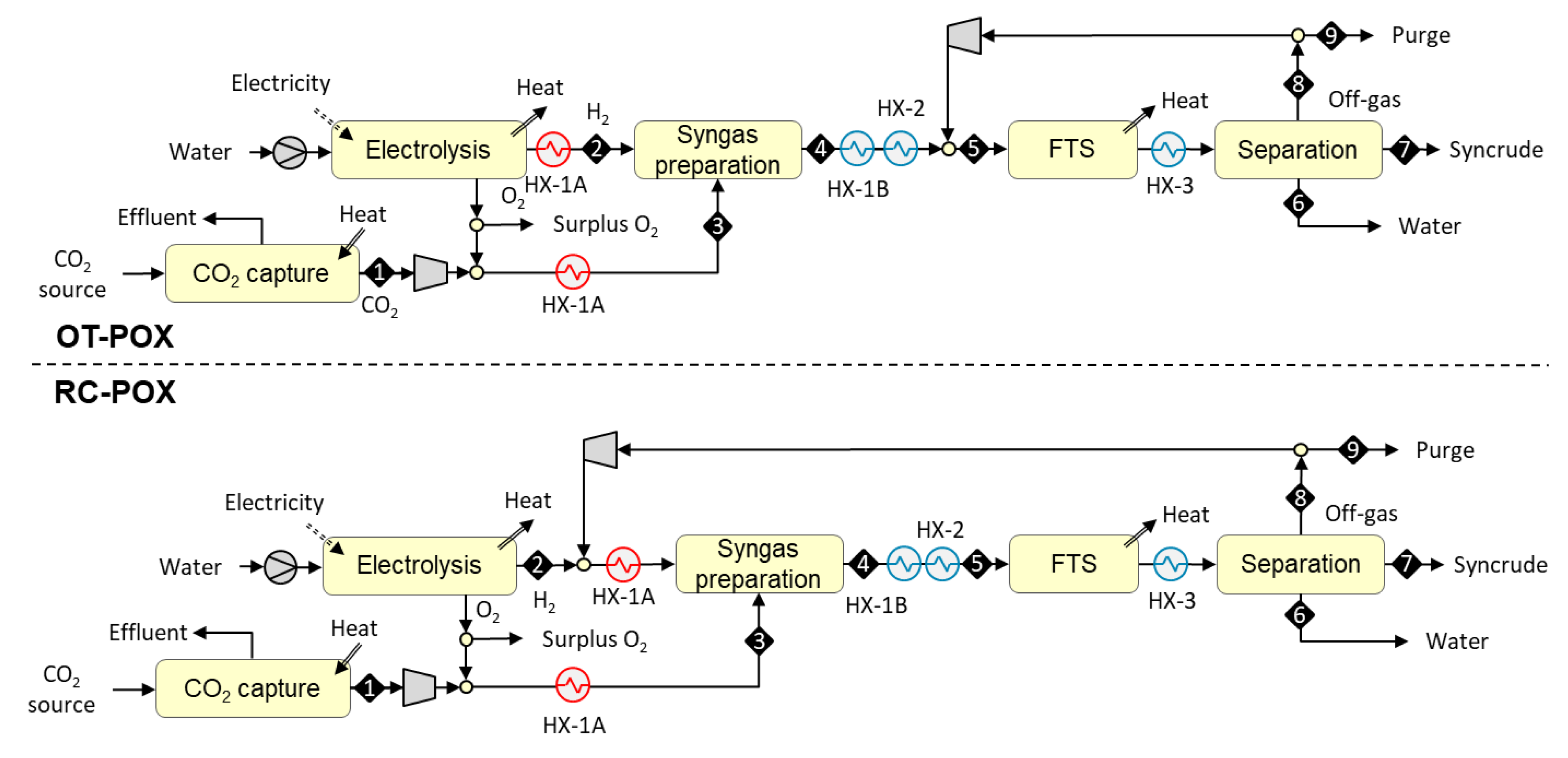

| Allothermal from Combustion | Autothermal from Partial Oxidation | Autothermal from Electric Resistance | |
|---|---|---|---|
| Once-through | OT-COMB | OT-POX | OT-ER |
| Recycle | RC-COMB | RC-POX | RC-ER |
| Item | Design Parameters |
|---|---|
| Electrolyser | H2 and O2 purity 100%, Both delivered at 20 bar and 25 °C. Nominal system efficiency from electricity to hydrogen is 60% (LHV) for AEC/PEM and 90% (LHV) for SOEC. |
| Syngas preparation reactor | Reactors modelled with RGibbs using Redlich-Kwong-Soave equation of state with Boston-Mathias modification (RKS-BM). Both phase and chemical equilibrium calculated. All components considered as products. Toutlet = 900 °C, ∆p = −0.5 bar. Target H2/CO ratio at the FT reactor inlet is 2.0 for all examined configurations. |
| Fischer-Tropsch synthesis | Treaction = 220 °C, Pfeed = 19–20 bar, ∆p =−0.5 bar, Boiling-water reactor using cobalt catalysts modelled with RStoic using Redlich-Kwong-Soave equation of state with Boston-Mathias modification (RKS-BM). Carbon monoxide reacts with hydrogen to form n-paraffins at 0.92 α value, with methane selectivity set to 9%. The per-pass CO conversion is set to 75%. Input H2O, CO2, N2 as well as methane, ethane and longer hydrocarbons are considered as inert. For RC-POX and RC-ER configurations, the amount of recycle is chosen to achieve 98% conversion of hydrogen (2% of hydrogen lost in purge). |
| Heat exchangers | ∆p = −0.1 bar, ∆T min = 15 °C (gas-liq), 30 °C (gas-gas). Heat loss = 1% of heat transferred. |
| Compressors | Stage pressure ratio < 2, ηpolytropic = 0.81, ηdriver = 0.90, ηmechanical = 0.98. Tintercooler = 35 °C, ∆p/pintercooler = 1%. |
| Pumps | ηhydraulic = 0.75, ηdriver = 0.90, ηmechanical = 0.98. |
| CONFIGURATION | OT-COMB | OT-POX | OT-ER | RC-COMB | RC-POX | RC-ER | |
|---|---|---|---|---|---|---|---|
| Feedstocks and intermediates | |||||||
| Captured and concentrated CO2 | kg/h | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Water for electrolysis | kg/h | 938 | 959 | 936 | 1092 | 1446 | 1216 |
| Electrolytic oxygen | kg/h | 833 | 852 | 831 | 969 | 1285 | 1080 |
| Electrolytic hydrogen | kg/h | 105 | 107 | 105 | 122 | 162 | 136 |
| MW | 3.5 | 3.6 | 3.5 | 4.1 | 5.4 | 4.5 | |
| Products | |||||||
| C5–C12 hydrocarbons | kg/h | 46 | 42 | 46 | 54 | 73 | 74 |
| MW | 0.6 | 0.5 | 0.6 | 0.7 | 0.9 | 0.9 | |
| C13+ hydrocarbons | kg/h | 131 | 120 | 130 | 154 | 208 | 211 |
| MW | 1.6 | 1.5 | 1.6 | 1.9 | 2.5 | 2.6 | |
| Total syncrude (C5+) | kg/h | 177 | 162 | 176 | 208 | 281 | 285 |
| MW | 2.2 | 2.0 | 2.1 | 2.5 | 3.4 | 3.5 | |
| Purge gas | kg/h | 422 | 356 | 97 | 77 | ||
| MW | 0.6 | 0.8 | 0.3 | 0.4 | |||
| Saturated steam | kg/h | 933 | 663 | 762 | 1097 | 1464 | 1500 |
| Condensed water | kg/h | 575 | 642 | 573 | 655 | 1015 | 774 |
| Surplus oxygen | kg/h | 833 | 733 | 831 | 969 | 1053 | 1080 |
| Electricity use | |||||||
| Electrolyser | |||||||
| Low temperature (AEC/PEM) | kW | 5830 | 5961 | 5814 | 6784 | 8989 | 7554 |
| High temperature (SOEC) | kW | 3887 | 3974 | 3876 | 4523 | 5993 | 5036 |
| Syngas preparation | kW | 334 | 774 | ||||
| Main compressor | kW | 74 | 73 | 73 | 77 | 73 | 73 |
| Recycle compressor | kW | 5 | 28 | 26 | 1 | 13 | 11 |
| Combustion air blower | kW | 10 | 12 | ||||
| Water pumps | kW | 1 | 1 | 1 | 2 | 2 | 2 |
| Sum total (AEC/PEM) | kW | 5920 | 6064 | 6249 | 6876 | 9077 | 8415 |
| Sum total (SOEC) | kW | 3976 | 4077 | 4311 | 4615 | 6081 | 5897 |
| Heat recovery | |||||||
| Electrolyser heat (AEC/PEM) | kW | 1846 | 1887 | 1841 | 2148 | 2 846 | 2 392 |
| Syngas cooling | kW | −21 | 274 | 136 | 194 | 418 | 264 |
| Syncrude cooling | kW | 636 | 684 | 610 | 561 | 877 | 671 |
| Flue gas cooling | kW | 404 | 388 | ||||
| FT reaction exotherm | kW | 660 | 469 | 539 | 777 | 1 036 | 1 062 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannula, I.; Kaisalo, N.; Simell, P. Preparation of Synthesis Gas from CO2 for Fischer–Tropsch Synthesis—Comparison of Alternative Process Configurations. C 2020, 6, 55. https://0-doi-org.brum.beds.ac.uk/10.3390/c6030055
Hannula I, Kaisalo N, Simell P. Preparation of Synthesis Gas from CO2 for Fischer–Tropsch Synthesis—Comparison of Alternative Process Configurations. C. 2020; 6(3):55. https://0-doi-org.brum.beds.ac.uk/10.3390/c6030055
Chicago/Turabian StyleHannula, Ilkka, Noora Kaisalo, and Pekka Simell. 2020. "Preparation of Synthesis Gas from CO2 for Fischer–Tropsch Synthesis—Comparison of Alternative Process Configurations" C 6, no. 3: 55. https://0-doi-org.brum.beds.ac.uk/10.3390/c6030055





