Electrochemical Aspects of a Nitrogen-Doped Pseudo-Graphitic Carbon Material: Resistance to Electrode Fouling by Air-Aging and Dopamine Electro-Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Nitrogen-Doped Pseudo-Graphite (N-GUITAR)
2.3. Scanning Electron Microscopy (SEM)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Raman
2.6. Powder X-ray Diffraction (XRD)
2.7. Water Contact Angle Measurement (WCA)
2.8. Electrode Fabrication and Electrochemistry
3. Results and Discussion
3.1. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Micrographs
3.3. Raman Analysis
3.4. X-ray Diffraction (XRD) Studies
3.5. Aqueous Potential Windows
3.6. Tafel Corrosion Studies
3.7. Heterogeneous Electron Transfer (HET) Characteristics of N-GUITAR with Fe(CN)63−/4−
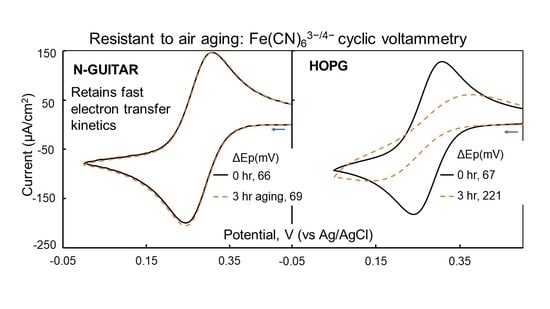
3.8. N-GUITAR Is Resistant to Air-Aging
3.9. N-Doped GUITAR Is Tolereant to Dopamine Fouling Because of Fast HET Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabir, H.; Zhu, H.; May, J.; Hamal, K.; Kan, Y.; Williams, T.; Echeverria, E.; McIlroy, D.N.; Estrada, D.; Davis, P.H.; et al. The sp2-sp3 carbon hybridization content of nanocrystalline graphite from pyrolyzed vegetable oil, comparison of electrochemistry and physical properties with other carbon forms and allotropes. Carbon 2019, 144, 831–840. [Google Scholar] [CrossRef]
- Gyan, I.O.; Wojcik, P.M.; Aston, D.E.; McIlroy, D.N.; Cheng, I.F. A Study of the Electrochemical Properties of a New Graphitic Material: GUITAR. ChemElectroChem 2015, 2, 700–706. [Google Scholar] [CrossRef]
- Cheng, I.F.; Xie, Y.; Allen Gonzales, R.; Brejna, P.R.; Sundararajan, J.P.; Fouetio Kengne, B.A.; Eric Aston, D.; McIlroy, D.N.; Foutch, J.D.; Griffiths, P.R. Synthesis of graphene paper from pyrolyzed asphalt. Carbon 2011, 49, 2852–2861. [Google Scholar] [CrossRef]
- Gyan, I.O.; Cheng, I.F. Electrochemical study of biologically relevant molecules at electrodes constructed from GUITAR, a new carbon allotrope. Microchem. J. 2015, 122, 39–44. [Google Scholar] [CrossRef]
- Kabir, H.; Ma, P.Y.; Renn, N.; Nicholas, N.W.; Cheng, I.F. Electrochemical determination of free chlorine on pseudo-graphite electrode. Talanta 2019, 205, 120101. [Google Scholar] [CrossRef]
- Kabir, H.; Zhu, H.; Lopez, R.; Nicholas, N.W.; McIlroy, D.N.; Echeverria, E.; May, J.; Cheng, I.F. Electrochemical determination of chemical oxygen demand on functionalized pseudo-graphite electrode. J. Electroanal. Chem. 2019, 113448. [Google Scholar] [CrossRef]
- Zhu, H.; Hassan, T.; Kabir, H.; May, J.; Hamal, K.; Lopez, R.J.; Smith, H.W.; Nicholas, N.; Sankaran, P.N.; McIlroy, D.; et al. Voltammetric pH sensor based on electrochemically modified pseudo-graphite. Analyst 2020. [Google Scholar] [CrossRef]
- Slate, A.J.; Brownson, D.A.C.; Dena, A.S.A.; Smith, G.C.; Whitehead, K.A.; Banks, C.E. Exploring the electrochemical performance of graphite and graphene paste electrodes composed of varying lateral flake sizes. Phys. Chem. Chem. Phys. 2018, 20, 20010–20022. [Google Scholar] [CrossRef] [Green Version]
- McCreery, R.L.; McDermott, M.T. Comment on Electrochemical Kinetics at Ordered Graphite Electrodes. Anal. Chem. 2012, 84, 2602–2605. [Google Scholar] [CrossRef]
- Davies, T.J.; Hyde, M.E.; Compton, R.G. Nanotrench Arrays Reveal Insight into Graphite Electrochemistry. Angew. Chem. Int. Ed. 2005, 44, 5121–5126. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Collignon, M.G.; O’Connell, M.A.; Hung, W.O.Y.; McKelvey, K.; Macpherson, J.V.; Unwin, P.R. A New View of Electrochemistry at Highly Oriented Pyrolytic Graphite. J. Am. Chem. Soc. 2012, 134, 20117–20130. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Li, Z.; Kozbial, A.; Nioradze, N.; Parobek, D.; Shenoy, G.J.; Salim, M.; Amemiya, S.; Li, L.; Liu, H. Water Protects Graphitic Surface from Airborne Hydrocarbon Contamination. ACS Nano 2016, 10, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.; Hurst, J.; Montgomery, M.; Tolman, N.; Liu, H. Airborne contamination of graphite as analyzed by ultra-violet photoelectron spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 2019, 235, 8–15. [Google Scholar] [CrossRef]
- Patel, A.N.; Tan, S.; Miller, T.S.; Macpherson, J.V.; Unwin, P.R. Comparison and Reappraisal of Carbon Electrodes for the Voltammetric Detection of Dopamine. Anal. Chem. 2013, 85, 11755–11764. [Google Scholar] [CrossRef]
- Trouillon, R.; O’Hare, D. Comparison of glassy carbon and boron doped diamond electrodes: Resistance to biofouling. Electrochim. Acta 2010, 55, 6586–6595. [Google Scholar] [CrossRef]
- Peltola, E.; Sainio, S.; Holt, K.B.; Palomäki, T.; Koskinen, J.; Laurila, T. Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes. Anal. Chem. 2018, 90, 1408–1416. [Google Scholar] [CrossRef] [Green Version]
- Puthongkham, P.; Venton, B.J. Nanodiamond Coating Improves the Sensitivity and Antifouling Properties of Carbon Fiber Microelectrodes. ACS Sens. 2019, 4, 2403–2411. [Google Scholar] [CrossRef]
- Bennet, K.E.; Tomshine, J.R.; Min, H.-K.; Manciu, F.S.; Marsh, M.P.; Paek, S.B.; Settell, M.L.; Nicolai, E.N.; Blaha, C.D.; Kouzani, A.Z.; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front. Hum. Neurosci 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; McDonald, A.G. Esterification of industrial lignin and its effect on the resulting poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or polypropylene blends. Ind. Crop. Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; Zeng, D.; Yu, X.; Xie, F.; Zhang, W.; Chen, J.; Yan, J.; Xie, F.; Wang, L.; Meng, H.; et al. Exploring the active sites of nitrogen-doped graphene as catalysts for the oxygen reduction reaction. Int. J. Hydrogen Energy 2014, 39, 15996–16005. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, L.; Yadav, R.M.; Yang, Y.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-Doped Graphene with Pyridinic Dominance as a Highly Active and Stable Electrocatalyst for Oxygen Reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar] [CrossRef]
- Wu, T.; Shen, H.; Sun, L.; Cheng, B.; Liu, B.; Shen, J. Nitrogen and boron doped monolayer graphene by chemical vapor deposition using polystyrene, urea and boric acid. New J. Chem. 2012, 36, 1385–1391. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; MacDonald, B.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Zan, R.; Altuntepe, A. Nitrogen doping of graphene by CVD. J. Mol. Struct. 2020, 1199, 127026. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Hu, Z.; Huo, K.; Ma, Y.; Chen, Y.; Wang, X.; Lu, Y. Synergism of C5N Six-Membered Ring and Vapor−Liquid−Solid Growth of CNx Nanotubes with Pyridine Precursor. J. Phys. Chem. B 2006, 110, 16422–16427. [Google Scholar] [CrossRef] [PubMed]
- Couzi, M.; Bruneel, J.-L.; Talaga, D.; Bokobza, L. A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited. Carbon 2016, 107, 388–394. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Hou, P.-X.; Unno, M.; Yamauchi, S.; Saito, R.; Kyotani, T. Dual Raman Features of Double Coaxial Carbon Nanotubes with N-Doped and B-Doped Multiwalls. Nano Lett. 2005, 5, 2465–2469. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Nitrogen- and Boron-Doped Double-Walled Carbon Nanotubes. ACS Nano 2007, 1, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Andonovic, B.; Temkov, M.; Ademi, A.; Petrovski, A. Laue functions model vs. scherrer equation in determination of graphene layers number on the ground of xrd data. J. Chem. Technol. Metall. 2014, 6, 545–550. [Google Scholar]
- Warren, B.E. X-Ray Diffraction in Random Layer Lattices. Phys. Rev. 1941, 59, 693–698. [Google Scholar] [CrossRef]
- Kamata, T.; Kato, D.; Niwa, O. Electrochemical performance at sputter-deposited nanocarbon film with different surface nitrogen-containing groups. Nanoscale 2019, 11, 10239–10246. [Google Scholar] [CrossRef]
- Tanaka, Y.; Furuta, M.; Kuriyama, K.; Kuwabara, R.; Katsuki, Y.; Kondo, T.; Fujishima, A.; Honda, K. Electrochemical properties of N-doped hydrogenated amorphous carbon films fabricated by plasma-enhanced chemical vapor deposition methods. Electrochim. Acta 2011, 56, 1172–1181. [Google Scholar] [CrossRef]
- Yang, X.; Haubold, L.; DeVivo, G.; Swain, G.M. Electroanalytical Performance of Nitrogen-Containing Tetrahedral Amorphous Carbon Thin-Film Electrodes. Anal. Chem. 2012, 84, 6240–6248. [Google Scholar] [CrossRef]
- Kamata, T.; Kato, D.; Ida, H.; Niwa, O. Structure and electrochemical characterization of carbon films formed by unbalanced magnetron (UBM) sputtering method. Diam. Relat. Mater. 2014, 49, 25–32. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Z.; Su, W.; Fan, X.; Liu, J.; Yan, C.; Zeng, C. Kinetic investigation of vanadium (V)/(IV) redox couple on electrochemically oxidized graphite electrodes. Electrochim. Acta 2016, 205, 102–112. [Google Scholar] [CrossRef]
- Chorbadzhiyska, E.; Bardarov, I.; Hubenova, Y.; Mitov, M. Graphite–Metal Oxide Composites as Potential Anodic Catalysts for Microbial Fuel Cells. Catalysts 2020, 10, 796. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. Determination of the Tafel slope for oxygen evolution on boron-doped diamond electrodes. Electrochem. Commun. 2008, 10, 607–610. [Google Scholar] [CrossRef]
- Suffredini, H.B.; Machado, S.A.S.; Avaca, L.A. The water decomposition reactions on boron-doped diamond electrodes. J. Braz. Chem. Soc. 2004, 15, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Goss, C.A.; Brumfield, J.C.; Irene, E.A.; Murray, R.W. Imaging the incipient electrochemical oxidation of highly oriented pyrolytic graphite. Anal. Chem. 1993, 65, 1378–1389. [Google Scholar] [CrossRef]
- Alliata, D.; Kötz, R.; Haas, O.; Siegenthaler, H. In Situ AFM Study of Interlayer Spacing during Anion Intercalation into HOPG in Aqueous Electrolyte. Langmuir 1999, 15, 8483–8489. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Huang, L.; Cao, Y.; Diao, D. Nanosized graphene sheets induced high electrochemical activity in pure carbon film. Electrochim. Acta 2018, 262, 173–181. [Google Scholar] [CrossRef]
- Chen, P.; McCreery, R.L. Control of Electron Transfer Kinetics at Glassy Carbon Electrodes by Specific Surface Modification. Anal. Chem. 1996, 68, 3958–3965. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Pham, T.; Ramnani, P.; Mulchandani, A. Carbon allotropes as sensors for environmental monitoring. Curr. Opin. Electrochem. 2017, 3, 106–113. [Google Scholar] [CrossRef]
- Kislenko, V.A.; Pavlov, S.V.; Kislenko, S.A. Influence of defects in graphene on electron transfer kinetics: The role of the surface electronic structure. Electrochim. Acta 2020, 341, 136011. [Google Scholar] [CrossRef]
- Kamata, T.; Kato, D.; Hirono, S.; Niwa, O. Structure and Electrochemical Performance of Nitrogen-Doped Carbon Film Formed by Electron Cyclotron Resonance Sputtering. Anal. Chem. 2013, 85, 9845–9851. [Google Scholar] [CrossRef]
- Lagrini, A.; Deslouis, C.; Cachet, H.; Benlahsen, M.; Charvet, S. Elaboration and electrochemical characterization of nitrogenated amorphous carbon films. Electrochem. Commun. 2004, 6, 245–248. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Zhang, J.; Jin, X.; Liu, J.-Y.; Li, Q.; Li, M.-H.; Cai, W.; Wu, D.-Y.; Zhan, D.; Ren, B. Quantitative Correlation between Defect Density and Heterogeneous Electron Transfer Rate of Single Layer Graphene. J. Am. Chem. Soc. 2014, 136, 16609–16617. [Google Scholar] [CrossRef]
- Ji, X.; Banks, C.E.; Crossley, A.; Compton, R.G. Oxygenated Edge Plane Sites Slow the Electron Transfer of the Ferro-/Ferricyanide Redox Couple at Graphite Electrodes. ChemPhysChem 2006, 7, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; et al. Effect of airborne contaminants on the wettability of supported graphene and graphite. Nat. Mater. 2013, 12, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, A.; Li, Z.; Sun, J.; Gong, X.; Zhou, F.; Wang, Y.; Xu, H.; Liu, H.; Li, L. Understanding the intrinsic water wettability of graphite. Carbon 2014, 74, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Kabir, H.; Lopez, R.; May, J.; Hamal, K.; Nicholas, N.W.; Cheng, I.F. Salicylic Acid Detection Using a Pseudo-Graphite Electrode. Curr. Top. Anal. Chem. 2019, 9, 1–8. [Google Scholar]
- Kamal Eddin, F.B.; Wing Fen, Y. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef] [Green Version]
- Harreither, W.; Trouillon, R.; Poulin, P.; Neri, W.; Ewing, A.G.; Safina, G. Carbon Nanotube Fiber Microelectrodes Show a Higher Resistance to Dopamine Fouling. Anal. Chem. 2013, 85, 7447–7453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, H.; Gyan, I.O.; Foutch, J.D.; Zhu, H.; Cheng, I.F. Application of GUITAR on the Negative Electrode of the Vanadium Redox Flow Battery: Improved V3+/2+ Heterogeneous Electron Transfer with Reduced Hydrogen Gassing. C J. Carbon Res. 2016, 2, 13. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamal, K.; May, J.; Zhu, H.; Dalbec, F.; Echeverria, E.; McIlroy, D.N.; Aston, E.; Cheng, I.F. Electrochemical Aspects of a Nitrogen-Doped Pseudo-Graphitic Carbon Material: Resistance to Electrode Fouling by Air-Aging and Dopamine Electro-Oxidation. C 2020, 6, 68. https://0-doi-org.brum.beds.ac.uk/10.3390/c6040068
Hamal K, May J, Zhu H, Dalbec F, Echeverria E, McIlroy DN, Aston E, Cheng IF. Electrochemical Aspects of a Nitrogen-Doped Pseudo-Graphitic Carbon Material: Resistance to Electrode Fouling by Air-Aging and Dopamine Electro-Oxidation. C. 2020; 6(4):68. https://0-doi-org.brum.beds.ac.uk/10.3390/c6040068
Chicago/Turabian StyleHamal, Kailash, Jeremy May, Haoyu Zhu, Forrest Dalbec, Elena Echeverria, David N. McIlroy, Eric Aston, and I. Francis Cheng. 2020. "Electrochemical Aspects of a Nitrogen-Doped Pseudo-Graphitic Carbon Material: Resistance to Electrode Fouling by Air-Aging and Dopamine Electro-Oxidation" C 6, no. 4: 68. https://0-doi-org.brum.beds.ac.uk/10.3390/c6040068