Structural Evolution of Polyimide-Derived Carbon during Phosphoric Acid Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursor Polymer
2.2. Synthesis of Carbon Adsorbents
2.3. Porous Structure
2.4. XPS
2.5. 31P MAS NMR
2.6. Acid-Base Properties
2.7. Copper Binding
3. Results and Discussion
3.1. Porous Structure
3.2. XPS
3.3. 31P MAS NMR
3.4. Acid-Base Properties
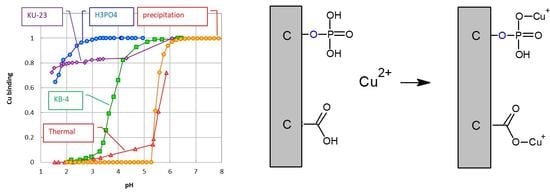
3.5. Copper Binding
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodríguez-Reinoso, F. Production and Applications of Activated Carbons. In Handbook of Porous Solids; Schüth, F., Sing, K.S.W., Weitkamp, J., Eds.; Wiley: Weinheim, Germany, 2002; Volume 3, pp. 1766–1827. ISBN 9783527618286. [Google Scholar]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier Ltd.: Oxford, UK, 2006; ISBN 978-0-08-044463-5. [Google Scholar]
- Menéndez-Díaz, J.A.; Martín-Gullón, I. Types of Carbon Adsorbents and Their Production. In Activated Carbon Surfaces in Environmental Remediation; Bandosz, T.J., Ed.; Interface Science and Technology; Academic Press: Amsterdam, The Netherlands, 2006; Volume 7, pp. 1–47. [Google Scholar]
- Kwiatkowski, J.F. (Ed.) Activated Carbon: Classifications, Properties and Applications; Nova Science Publishers: New York, NY, USA, 2012; Volume 66, ISBN 9781620816660. [Google Scholar]
- Henning, K.-D.; von Kienle, H. Carbon, 5. Activated Carbon. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 6, pp. 771–796. [Google Scholar]
- Çeçen, F. Activated Carbon. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–34. [Google Scholar]
- Rodriguez-Reinoso, F.; Silvestre-Albero, J. Activated Carbon and Adsorption. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–14. ISBN 9780128035818. [Google Scholar]
- Macías-García, A.; Díaz-Díez, M.A.; Gómez-Serrano, V.; González, M.C.F. Preparation and Characterization of Activated Carbons Made up from Different Woods by Chemical Activation with H3PO4. Smart Mater. Struct. 2003, 12, N24–N28. [Google Scholar] [CrossRef]
- Muñoz, Y.; Arriagada, R.; Soto-Garrido, G.; García, R. Phosphoric and Boric Acid Activation of Pine Sawdust. J. Chem. Technol. Biotechnol. 2003, 78, 1252–1258. [Google Scholar] [CrossRef]
- Attia, A.A.; Girgis, B.S.; Khedr, S.A. Capacity of Activated Carbon Derived from Pistachio Shells by H3PO4 in the Removal of Dyes and Phenolics. J. Chem. Technol. Biotechnol. 2003, 78, 611–619. [Google Scholar] [CrossRef]
- Diao, Y.; Walawender, W.P.; Fan, L.T. Activated Carbons Prepared from Phosphoric Acid Activation of Grain Sorghum. Bioresour. Technol. 2002, 81, 45–52. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-Containing Carbons: Preparation, Properties and Utilization. Carbon 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Benaddi, H.; Legras, D.; Rouzaud, J.-N.; Béguin, F. Influence of the Atmosphere in the Chemical Activation of Wood by Phosphoric Acid. Carbon 1998, 36, 306–309. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X. Peanut Shell Activated Carbon: Characterization, Surface Modification and Adsorption of Pb2+ from Aqueous Solution. Chin. J. Chem. Eng. 2008, 16, 401–406. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Ziatdinov, A.M. On the Chemical Structure of Phosphorus Compounds in Phosphoric Acid-Activated Carbon. Appl. Surf. Sci. 2006, 252, 8036–8038. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wiśniewski, M. XPS and NMR Studies of Phosphoric Acid Activated Carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Huang, C.; Sun, T.; Hulicova-Jurcakova, D. Wide Electrochemical Window of Supercapacitors from Coffee Bean-Derived Phosphorus-Rich Carbons. ChemSusChem 2013, 6, 2330–2339. [Google Scholar] [CrossRef]
- Castro-Muñiz, A.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Activated Carbon Fibers with a High Content of Surface Functional Groups by Phosphoric Acid Activation of PPTA. J. Colloid Interface Sci. 2011, 361, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, D.; Gutiérrez, M.C.; Picó, F.; Rojo, J.M.; Fierro, J.L.G.; Ferrer, M.L.; del Monte, F. Phosphate-Functionalized Carbon Monoliths from Deep Eutectic Solvents and Their Use as Monolithic Electrodes in Supercapacitors. ChemSusChem 2012, 5, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Bairi, V.G.; Bourdo, S.E.; Nasini, U.B.; Ramasahayam, S.K.; Watanabe, F.; Berry, B.C.; Viswanathan, T. Microwave-Assisted Synthesis of Nitrogen and Phosphorus Co-Doped Mesoporous Carbon and Their Potential Application in Alkaline Fuel Cells. Sci. Adv. Mater. 2013, 5, 1275–1281. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and Characterization of Activated Carbon from Reedy Grass Leaves by Chemical Activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Fu, R.; Liu, L.; Huang, W.; Sun, P. Studies on the Structure of Activated Carbon Fibers Activated by Phosphoric Acid. J. Appl. Polym. Sci. 2003, 87, 2253–2261. [Google Scholar] [CrossRef]
- Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. A Kinetic Study of 2-Propanol Dehydration on Carbon Acid Catalysts. J. Catal. 2010, 271, 33–42. [Google Scholar] [CrossRef]
- Bedia, J.; Barrionuevo, R.; Rodríguez-Mirasol, J.; Cordero, T. Ethanol Dehydration to Ethylene on Acid Carbon Catalysts. Appl. Catal. B Environ. 2011, 103, 302–310. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of the Oxidation Resistance of Phosphorus-Containing Activated Carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amorós, D.; Morallón, E.; Nishihara, H.; Kyotani, T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced Electro-Oxidation Resistance of Carbon Electrodes Induced by Phosphorus Surface Groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Berenguer, R.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Phosphorus Functionalization for the Rapid Preparation of Highly Nanoporous Submicron-Diameter Carbon Fibers by Electrospinning of Lignin Solutions. J. Mater. Chem. A 2018, 6, 1219–1233. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, S.; Yang, J.; Yoon, S.-H. Evolution of Phosphorus-Containing Groups on Activated Carbons during Heat Treatment. Langmuir 2017, 33, 3112–3122. [Google Scholar] [CrossRef]
- Tebby, J.C. (Ed.) Handbook of Phosphorus. 31 Nuclear Magnetic Resonance Data (1990); CRC Press: Boca Raton, FL, USA, 2017; ISBN 9780203712122. [Google Scholar]
- Potrzebowski, M.J.; Kaźmierski, S.; Kassassir, H.; Miksa, B. Phosphorus-31 NMR Spectroscopy of Condensed Matter. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 70, pp. 35–114. ISBN 9780123813534. [Google Scholar]
- Jagtoyen, M.; Thwaites, M.; Stencel, J.M.; McEnaney, B.; Derbyshire, F. Adsorbent Carbon Synthesis from Coals by Phosphoric Acid Activation. Carbon 1992, 30, 1089–1096. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Pacholczyk, A.; Terzyk, A.P.; Rychlicki, G. New Phosphorus-Containing Spherical Carbon Adsorbents as Promising Materials in Drug Adsorption and Release. J. Colloid Interface Sci. 2011, 354, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Sánchez, M.; Primo, A.; García, H. P-Doped Graphene Obtained by Pyrolysis of Modified Alginate as a Photocatalyst for Hydrogen Generation from Water-Methanol Mixtures. Angew. Chem. Int. Ed. 2013, 52, 11813–11816. [Google Scholar] [CrossRef]
- MacIntosh, A.R.; Jiang, G.; Zamani, P.; Song, Z.; Riese, A.; Harris, K.J.; Fu, X.; Chen, Z.; Sun, X.; Goward, G.R. Phosphorus and Nitrogen Centers in Doped Graphene and Carbon Nanotubes Analyzed through Solid-State NMR. J. Phys. Chem. C 2018, 122, 6593–6601. [Google Scholar] [CrossRef]
- Das, T.K.; Banerjee, S.; Sharma, P.; Sudarsan, V.; Sastry, P.U. Nitrogen Doped Porous Carbon Derived from EDTA: Effect of Pores on Hydrogen Storage Properties. Int. J. Hydrogen Energy 2018, 43, 8385–8394. [Google Scholar] [CrossRef]
- Albero, J.; Vidal, A.; Migani, A.; Concepción, P.; Blancafort, L.; García, H. Phosphorus-Doped Graphene as a Metal-Free Material for Thermochemical Water Reforming at Unusually Mild Conditions. ACS Sustain. Chem. Eng. 2019, 7, 838–846. [Google Scholar] [CrossRef]
- Singh, A.S.; Advani, J.H.; Biradar, A.V. Phosphonate Functionalized Carbon Spheres as Brønsted Acid Catalysts for the Valorization of Bio-Renewable α-Pinene Oxide to Trans -Carveol. Dalton Trans. 2020, 49, 7210–7217. [Google Scholar] [CrossRef]
- Matynia, T.; Gawdzik, B.; Chmielewska, E. Synthesis and Properties of Porous Copolymers of 4,4’-Bismaleimido Diphenyl Methane and Styrene. J. Appl. Polym. Sci. 1996, 60, 1971–1975. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Sobiesiak, M.; Dziadko, D. Heterogeneity of Synthetic Carbons Obtained from Polyimides. Appl. Surf. Sci. 2002, 196, 89–97. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Sobiesiak, M.; Tsyba, M.M. Functionalization of Carbon and Silica Gel by Phosphoric Acid. Adsorpt. Sci. Technol. 2007, 25, 531–542. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Sobiesiak, M.; Gawdzik, B. Structural and Surface Heterogeneity of Phosphorus-Containing Polyimide-Derived Carbons: Effect of Heat Treatment Temperature. Adsorption 2013, 19, 717–722. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Gawdzik, B.; Puziy, A.M.; Poddubnaya, O.I. Phosphoric Acid and Steam as Activation Agents for Carbonized Porous Polymer Surfaces. Adsorpt. Sci. Technol. 2006, 24, 167–176. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Gawdzik, B.; Puziy, A.M.; Poddubnaya, O.I. Polymer-Based Carbon Adsorbents Obtained from Copolymer of 4,4’-Bis(Maleimidodiphenyl)Methane and Divinylbenzene for Use in SPE. Chromatographia 2006, 64, 175–181. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. A Simple Two-Dimensional NLDFT Model of Gas Adsorption in Finite Carbon Pores. Application to Pore Structure Analysis. J. Phys. Chem. C 2009, 113, 19382–19385. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT Adsorption Models for Carbon Slit-Shaped Pores with Surface Energetical Heterogeneity and Geometrical Corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon Slit Pore Model Incorporating Surface Energetical Heterogeneity and Geometrical Corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET Equation Applicable to Microporous Adsorbents. In COPS-7: Characterization of Porous Solids VII; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy, 2nd ed.; Chastain, J., Ed.; Perkin-Elmer: Eden Prarie, MN, USA, 1992; ISBN 9780962702624. [Google Scholar]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD, 20899. 2000. Available online: https://0-doi-org.brum.beds.ac.uk/10.18434/T4T88K (accessed on 26 August 2022).
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 EV. J. Electron Spectros. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Lützenkirchen, J.; Preočanin, T.; Kovačević, D.; Tomišić, V.; Lövgren, L.; Kallay, N. Potentiometric Titrations as a Tool for Surface Charge Determination. Croat. Chem. Acta 2012, 85, 391–417. [Google Scholar] [CrossRef]
- del Piero, S.; Melchior, A.; Polese, P.; Portanova, R.; Tolazzi, M. A Novel Multipurpose Excel Tool for Equilibrium Speciation Based on Newton-Raphson Method and on a Hybrid Genetic Algorithm. Ann. Chim. 2006, 96, 29–49. [Google Scholar] [CrossRef]
- Provencher, S.W. A Constrained Regularization Method for Inverting Data Represented by Linear Algebraic or Integral Equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A General Purpose Constrained Regularization Program for Inverting Noisy Linear Algebraic and Integral Equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Puziy, A.M.; Matynia, T.; Gawdzik, B.; Poddubnaya, O.I. Use of CONTIN for Calculation of Adsorption Energy Distribution. Langmuir 1999, 15, 6016–6025. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Ritter, J.A.; Ebner, A.D.; Holland, C.E. Elucidation of the Ion Binding Mechanism in Heterogeneous Carbon-Composite Adsorbents. Carbon 2001, 39, 2313–2324. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Myglovets, M.; Poddubnaya, O.I.; Sevastyanova, O.; Lindström, M.E.; Gawdzik, B.; Sobiesiak, M.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Preparation of Carbon Adsorbents from Lignosulfonate by Phosphoric Acid Activation for the Adsorption of Metal Ions. Carbon 2014, 80, 771–783. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Sobiesiak, M.; Gawdzik, B. Assessment of the Structural Evolution of Polyimide-Derived Carbons Obtained by Phosphoric Acid Activation Using Fourier Transform Infrared and Raman Spectroscopy. Adsorpt. Sci. Technol. 2017, 35, 403–412. [Google Scholar] [CrossRef]
- Jackson, S.T.; Nuzzo, R.G. Determining Hybridization Differences for Amorphous Carbon from the XPS C 1s Envelope. Appl. Surf. Sci. 1995, 90, 195–203. [Google Scholar] [CrossRef]
- Haerle, R.; Riedo, E.; Pasquarello, A.; Baldereschi, A. Sp2/Sp3 Hybridization Ratio in Amorphous Carbon from C 1s Core-Level Shifts: X-ray Photoelectron Spectroscopy and First-Principles Calculation. Phys. Rev. B 2001, 65, 32–37. [Google Scholar] [CrossRef]
- Pandiyan, R.; Delegan, N.; Dirany, A.; Drogui, P.; El Khakani, M.A. Correlation of Sp2 Carbon Bonds Content in Magnetron-Sputtered Amorphous Carbon Films to Their Electrochemical H2O2 Production for Water Decontamination Applications. Carbon 2015, 94, 988–995. [Google Scholar] [CrossRef]
- Mominuzzaman, S.M.; Alam, M.; Soga, T.; Jimbo, T. Rearrangements of Sp2/Sp3 Hybridized Bonding with Phosphorus Incorporation in Pulsed Laser Deposited Semiconducting Carbon Films by X-ray Photoelectron Spectroscopic Analysis. Diam. Relat. Mater. 2006, 15, 1795–1798. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Rose, K.D.; Kwiatek, P.J. Carbon Aromaticity Based on XPS II to II∗ Signal Intensity. Appl. Surf. Sci. 1993, 64, 167–174. [Google Scholar] [CrossRef]
- Gardella, J.A.; Ferguson, S.A.; Chin, R.L. Π* ← π Shakeup Satellites for the Analysis of Structure and Bonding in Aromatic Polymers by X-ray Photoelectron Spectroscopy. Appl. Spectrosc. 1986, 40, 224–232. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Poddubnaya, O.I.; Tsyba, M.M.; Zakutevskyy, O.; Khyzhun, O.; Khalameida, S.; Puziy, A.M. Photocatalytic Degradation of Dyes Using Phosphorus-Containing Activated Carbons. Appl. Surf. Sci. 2021, 535, 147667. [Google Scholar] [CrossRef]
- Delobel, R.; Le Bras, M.; Ouassou, N.; Descressain, R. Fire Retardance of Polypropylene by Diammonium Pyrophosphate-Pentaerythritol: Spectroscopic Characterization of the Protective Coatings. Polym. Degrad. Stab. 1990, 30, 41–56. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R. Carbonization Mechanisms Resulting from Intumescence Association with the Ammonium Polyphosphate-Pentaerythritol Fire Retardant System. Carbon 1993, 31, 1219–1230. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Trémillon, J.-M. Synergistic Effect of Zeolite in an Intumescence Process. Study of the Interactions between the Polymer and the Additives. J. Chem. Soc. Faraday Trans. 1996, 92, 3435–3444. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Decressain, R.; Amoureux, J.-P. Synergistic Effect of Zeolite in an Intumescence Process: Study of the Carbonaceous Structures Using Solid-State NMR. J. Chem. Soc. Faraday Trans. 1996, 92, 149–158. [Google Scholar] [CrossRef]
- Krawietz, T.R.; Lin, P.; Lotterhos, K.E.; Torres, P.D.; Barich, D.H.; Clearfield, A.; Haw, J.F. Solid Phosphoric Acid Catalyst: A Multinuclear NMR and Theoretical Study. J. Am. Chem. Soc. 1998, 120, 8502–8511. [Google Scholar] [CrossRef]
- Glonek, T.; Van Wazer, J.R. Phosphorus-31 Spin-Lattice Relaxation of Esters of Orthophosphoric Acid. J. Phys. Chem. 1976, 80, 639–643. [Google Scholar] [CrossRef]
- Hayashi, S.; Hayamizu, K. High-Resolution Solid-State 31P NMR of Alkali Phosphates. Bull. Chem. Soc. Jpn. 1989, 62, 3061–3068. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated Carbons from Yellow Poplar and White Oak by H3PO4 Activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Harris, R.K.; Merwin, L.H.; Hägele, G. Corrigendum to Solid-State Nuclear Magnetic Resonance Study of a Series of Phosphonic and Phosphinic Acids. J. Chem. Soc. Faraday Trans. 1 1989, 85, 3899–3900. [Google Scholar] [CrossRef]
- Westall, J. Chemical Equilibrium Including Adsorption on Charged Surfaces. In Particulates in Water; Kavanaugh, M.C., Leckie, J.O., Eds.; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1980; Volume 189, pp. 33–44. ISBN 0-8412-0499-3. [Google Scholar]
- Puziy, A.M.; Poddubnaya, O.I.; Zaitsev, V.N.; Konoplitska, O.P. Modeling of Heavy Metal Ion Binding by Phosphoric Acid Activated Carbon. Appl. Surf. Sci. 2004, 221, 421–429. [Google Scholar] [CrossRef]
- Lützenkirchen, J. (Ed.) Surface Complexation Modelling; Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 11, ISBN 978-0-12-372572-1. [Google Scholar]
- Goldberg, S. Surface Complexation Modeling. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–14. ISBN 9780124095489. [Google Scholar]
- Zhang, Y.; Luo, W. Adsorptive Removal of Heavy Metal from Acidic Wastewater with Biochar Produced from Anaerobically Digested Residues: Kinetics and Surface Complexation Modeling. BioResources 2014, 9, 2484–2499. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J. Adsorbent−adsorbate Interactions in the Adsorption of Cd(II) and Hg(II) on Ozonized Activated Carbons. Environ. Sci. Technol. 2002, 36, 3850–3854. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M. Adsorption of Cr(III) on Ozonised Activated Carbon. Importance of Cπ—Cation Interactions. Water Res. 2003, 37, 3335–3340. [Google Scholar] [CrossRef]
- Leon y Leon, C.A.; Solar, J.M.; Calemma, V.; Radovic, L.R. Evidence for the Protonation of Basal Plane Sites on Carbon. Carbon 1992, 30, 797–811. [Google Scholar] [CrossRef]
- Álvarez-Merino, M.A.; López-Ramón, M.V.; Moreno-Castilla, C. A Study of the Static and Dynamic Adsorption of Zn(II) Ions on Carbon Materials from Aqueous Solutions. J. Colloid Interface Sci. 2005, 288, 335–341. [Google Scholar] [CrossRef]
- Montes-Morán, M.A.; Menéndez, J.A.; Fuente, E.; Suárez, D. Contribution of the Basal Planes to Carbon Basicity: An Ab Initio Study of the H3O+−π Interaction in Cluster Models. J. Phys. Chem. B 1998, 102, 5595–5601. [Google Scholar] [CrossRef]










| Temperature °C | ABET m2/g | Vtot cm3/g | Vmi cm3/g | Vme cm3/g |
|---|---|---|---|---|
| BM-DVB | ||||
| 55.9 | 0.338 | 0.006 (2%) | 0.332 (98%) | |
| H3PO4 series | ||||
| 400 | 11.5 | 0.026 | 0.002 (9%) | 0.023 (91%) |
| 500 | 520.2 | 0.263 | 0.197 (75%) | 0.066 (25%) |
| 600 | 891.1 | 0.422 | 0.324 (77%) | 0.098 (23%) |
| 700 | 676.3 | 0.336 | 0.259 (77%) | 0.078 (23%) |
| 800 | 599.4 | 0.316 | 0.228 (72%) | 0.088 (28%) |
| 900 | 659.7 | 0.337 | 0.255 (76%) | 0.082 (24%) |
| 1000 | 601.9 | 0.320 | 0.231 (72%) | 0.090 (28%) |
| Thermal series | ||||
| 400 | 36.5 | 0.228 | 0.004 (2%) | 0.223 (98%) |
| 500 | 37.5 | 0.182 | 0.006 (3%) | 0.176 (97%) |
| 600 | 491.3 | 0.349 | 0.174 (50%) | 0.175 (50%) |
| 700 | 522.0 | 0.328 | 0.194 (59%) | 0.134 (41%) |
| 800 | 356.5 | 0.199 | 0.129 (65%) | 0.070 (35%) |
| 900 | 223.9 | 0.171 | 0.080 (47%) | 0.090 (53) |
| 1000 | 20.3 | 0.073 | 0.002 (2%) | 0.071 (98) |
| Temperature °C | H3PO4 Series | Thermal Series | |||||
|---|---|---|---|---|---|---|---|
| C | O | N | P | C | O | N | |
| 400 | 81.2 | 12.6 | 2.6 | 2.2 | 79.7 | 15 | 5.3 |
| 500 | 74.2 | 15.2 | 2.3 | 6.6 | 84.2 | 10.4 | 5.4 |
| 600 | 74.5 | 12.3 | 1.6 | 11.6 | 91.1 | 4.3 | 4.6 |
| 700 | 68.9 | 15 | 3.7 | 12.4 | 93.2 | 2.9 | 3.9 |
| 800 | 66.1 | 17.1 | 3.6 | 13.2 | 93.3 | 3.4 | 3.3 |
| 900 | 68.6 | 17.3 | 3.4 | 10.6 | 94.4 | 3 | 2.6 |
| 1000 | 75.8 | 14.1 | 1.7 | 8.3 | 92 | 3.9 | 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Sobiesiak, M.; Sprynskyy, M. Structural Evolution of Polyimide-Derived Carbon during Phosphoric Acid Activation. C 2022, 8, 47. https://0-doi-org.brum.beds.ac.uk/10.3390/c8030047
Puziy AM, Poddubnaya OI, Gawdzik B, Sobiesiak M, Sprynskyy M. Structural Evolution of Polyimide-Derived Carbon during Phosphoric Acid Activation. C. 2022; 8(3):47. https://0-doi-org.brum.beds.ac.uk/10.3390/c8030047
Chicago/Turabian StylePuziy, Alexander M., Olga I. Poddubnaya, Barbara Gawdzik, Magdalena Sobiesiak, and Myroslav Sprynskyy. 2022. "Structural Evolution of Polyimide-Derived Carbon during Phosphoric Acid Activation" C 8, no. 3: 47. https://0-doi-org.brum.beds.ac.uk/10.3390/c8030047