Correlation of the Grapevine (Vitis vinifera L.) Leaf Chlorophyll Concentration with RGB Color Indices
Abstract
:1. Introduction
2. Materials and Methods
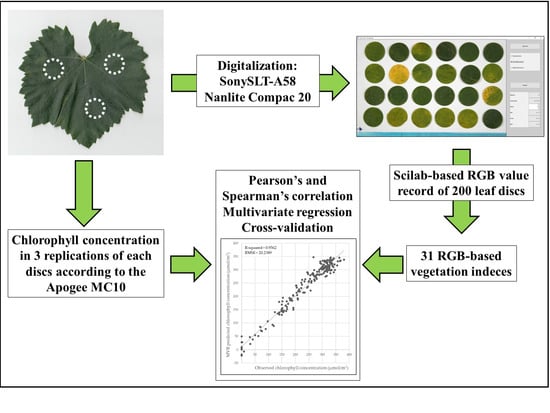
2.1. Sampling and Digitalization
2.2. Chlorophyll Concentration Measurement
2.3. Image Analysis and RGB-Based Color Index Calculation
2.4. Statistical Analysis
3. Results and Discussion
| Index | Formula | Present Study | [27] | [28] | [29] | [30] | [31] | [32] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s Corr. | Spearman’s Corr. | SD MVR | † | †† | |||||||
| Red—R | 0–255 | −0.9468 ** | −0.8942 ** | 0.2027 | - | + | + | + | - | - | - |
| Green—G | 0–255 | −0.9678 ** | −0.9109 ** | 0.2214 | - | + | + | + | + | - | - |
| Blue—B | 0–255 | 0.0501 | 0.4790 ** | 0.2013 | - | + | + | + | - | - | + |
| Red chromaticity—r | R/(R + G + B) | −0.9431 ** | −0.8723 ** | 0.0003 | - | + | - | - | - | ||
| Green chromaticity—g | G/(R + G + B) | −0.0079 | −0.4178 ** | 0.0007 | - | - | - | + | |||
| Blue chromaticity—b | B/(R + G + B) | 0.8805 ** | 0.8898 ** | 0.0009 | + | + | + | + | |||
| RMG (Difference between red and green) | R − G | −0.7832 ** | −0.4217 ** | 0.2663 | - | + | + | - | |||
| RMB (Difference between red and blue) | R − B | −0.9656 ** | −0.9183 ** | 0.1041 | - | - | - | - | |||
| GMB (Difference between green and blue) | G − B | −0.9656 ** | −0.9183 ** | 0.1041 | - | - | - | - | |||
| NRGVI (Normalized red-green difference index) | (R − G)/(R + G) | −0.8921 ** | −0.6729 ** | 0 | - | + | - | - | - | ||
| NRBVI (Normalized red-blue difference index) | (R − B)/(R + B) | −0.9043 ** | −0.8931 ** | 0.0036 | - | - | - | + | - | ||
| NGBVI (Normalized green-blue difference index) | (G − B)/(G + B) | −0.8437 ** | −0.8810 ** | 0.0027 | - | - | - | - | - | ||
| (R − G)/(R + G + B) | (R − G)/(R + G + B) | −0.8734 ** | −0.6042 ** | 0.0004 | - | - | - | - | |||
| (R − B)/(R + G + B) | (R − B)/(R + G + B) | −0.9271 ** | −0.8966 ** | 0.0012 | - | - | - | - | |||
| (G − B)/(R + G + B) | (G − B)/(R + G + B) | −0.7453 ** | −0.8412 ** | 0.0016 | - | - | - | - | |||
| RGRI (Red-Green Ratio Index) | R/G | −0.8838 ** | −0.6729 ** | 0.3204 | + | + | - | - | |||
| GLI (Green leaf index) | (2G − R − B)/(2G + R + B) | 0.0045 | −0.4178 ** | 0.0567 | + | ||||||
| VARI (Visible atmospherically resistance index) | (G − R)/(G + R − B) | 0.9160 ** | 0.7482 ** | 0.3116 | + | ||||||
| IPCA | 0.994|R − B| + 0.961|G − B| + 0.914|G − R| | −0.9671 ** | −0.9182 ** | 0.1879 | - | ||||||
| ExR (Excess red vegetation index) | 1.4r − g | −0.8734 ** | −0.6042 ** | 0.0006 | - | ||||||
| ExB (Excess blue vegetation index) | 1.4b − g | 0.7453 ** | 0.8412 ** | 0.0022 | + | ||||||
| ExG (Excess green vegetation index) | 2g − r − b | −0.9431 ** | −0.8723 ** | 0.0013 | + | ||||||
| ExGR (Excess green minus Excess red) | ExG − ExR | −0.9244 ** | −0.8972 ** | 0.0017 | + | ||||||
| Gray | 0.2898r + 0.5870g + 0.1140b | −0.6522 ** | −0.7845 ** | 0.0004 | - | ||||||
| CIVE (Color Index for Vegetation Extraction) | 0.441r − 0.811g + 0.385b + 18.78 | −0.103 | 0.3466 ** | 0.0008 | - | ||||||
| PCA1 (Principal Compoment Analysis 1) | −0.977b + 0.916((G − B)/(G + B)) + 0.995((R − B)/(R + B)) + 0.771((R − G)/(R + G)) | −0.9060 ** | −0.8940 ** | 0.007 | - | ||||||
| PCA2 (Principal Compoment Analysis 2) | 0.999|R − B| + 0.92|G − B| + 0.886|R − G| | −0.9709 ** | −0.9163 ** | 0.1569 | - | ||||||
| I1 | R + G − 2B | −0.9706 ** | −0.9162 ** | 0.1755 | - | ||||||
| SLR1 (Stepwise Linear Regression 1) | −60.430 − 0.7316B + 69.680b + 112.800g + 28.270((G − B)/(G + B)) − 23.890((R − B)/(R + B)) + 68.380((R − G)/(R + G)) | −0.8920 ** | −0.6728 ** | 0.3164 | + | ||||||
| SLR2 (Stepwise Linear Regression 2) | −46.240 − 2.678B + 1.05G + 52.570b + 87.420g + 20.720((G − B)/(G + B)) − 18.240((R − B)/(R + B)) + 52.500((R − G)/(R + G)) | −0.1812 * | 0.2924 ** | 0.3849 | + | ||||||
| SLR3 (Stepwise Linear Regression 3) | −25.373 + 30.106b + 46.539g + 12776((G − B)/(G + B)) − 10.507((R − B)/(R + B)) + 28.821((R − G)/(R + G)) | −0.2822 ** | 0.2148 ** | 0.2778 | + | ||||||
| SLR4 (Stepwise Linear Regression 4) | −44.312 + 51.689b + 81.995g + 21.751((G − B)/(G + B)) − 18.156((R − B)/(R + B)) + 50.425((R − G)/(R + G)) | −0.4961 ** | −0.1038 | 0.3728 | + | ||||||
| SLR5 (Stepwise Linear Regression 5) | −41.048 + 46.964b + 76.841g + 19.998((G − B)/(G + B)) − 17.173((R − B)/(R + B)) + 47.162((R − G)/(R + G)) | −0.4242 ** | −0.0289 | 0.36 | + | ||||||
| I2 | 0.55 + 11.4((G − B)/(G + B)) − 12.5((R − B)/(R + B)) + 9((R − G)/(R + G)) | 0.7945 ** | 0.8455 ** | 0.0156 | + | ||||||
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammoniaci, M.; Kartsiotis, S.P.; Perria, R.; Storchi, P. State of the art of monitoring technologies and data processing for precision viticulture. Agriculture 2021, 11, 201. [Google Scholar] [CrossRef]
- De Bei, R.; Fuentes, S.; Gilliham, M.; Tyerman, S.; Edwards, E.; Bianchini, N.; Collins, C. VitiCanopy: A free computer App to estimate canopy vigor and porosity for grapevine. Sensors 2019, 16, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briglia, N.; Montanaro, G.; Petrozza, A.; Summerer, S.; Cellini, F.; Nuzzo, V. Drought phenotyping in Vitis vinifera using RGB and NIR imaging. Sci. Hortic. 2019, 256, 108555. [Google Scholar] [CrossRef]
- Acevedo-Opazo, C.; Tisseyre, B.; Guillaume, S.; Ojeda, H. Test of NDVI information for a relevant vineyard zoning related to vine water status. In Proceedings of the 6th ECPA-European Conference on Precision Agriculture, Skiathos, Greece, 3–6 June 2007; pp. 547–554. [Google Scholar]
- Palacios, F.; Diago, M.P.; Melo-Pinto, P.; Tardaguila, J. Early yield prediction in different grapevine varieties using computer vision and machine learning. Precis. Agric. 2022, 24, 407–435. [Google Scholar] [CrossRef]
- Arnó, J.; Martínez Casasnovas, J.A.; Ribes Dasi, M.; Rosell Polo, J.R. Precision viticulture. Research topics, challenges and opportunities in site-specific vineyard management. Span. J. Agric. Res. 2009, 7, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Matese, A.; Di Gennaro, S.F. Technology in precision viticulture: A state of the art review. Int. J. Wine Res. 2015, 7, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Seng, K.P.; Ang, L.M.; Schmidtke, L.M.; Rogiers, S.Y. Computer vision and machine learning for viticulture technology. IEEE Access 2018, 6, 67494–67510. [Google Scholar] [CrossRef]
- Bodor-Pesti, P.; Taranyi, D.; Deák, T.; Nyitrainé Sárdy, D.Á.; Varga, Z. A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf. Plants 2023, 12, 452. [Google Scholar] [CrossRef]
- Angulo, O.; Fidelibus, M.W.; Heymann, H. Grape cultivar and drying method affect sensory characteristics and consumer preference of raisins. J. Sci. Food Agric. 2007, 87, 865–870. [Google Scholar] [CrossRef]
- Chironi, S.; Sortino, G.; Allegra, A.; Saletta, F.; Caviglia, V.; Ingrassia, M. Consumer assessment on sensory attributes of fresh table grapes cv “Italia” and “Red Globe” after long cold storage treatment. Chem. Eng. Trans. 2017, 58, 421–426. [Google Scholar]
- Baofeng, S.; Jinru, X.; Chunyu, X.; Yuyang, S.; Fuentes, S. Digital surface model applied to unmanned aerial vehicle based photogrammetry to assess potential biotic or abiotic effects on grapevine canopies. Int. J. Agric. Biol. Eng. 2016, 9, 119–130. [Google Scholar]
- Diago, M.P.; Aquino, A.; Millan, B.; Palacios, F.; Tardáguila, J. On-the-go assessment of vineyard canopy porosity, bunch and leaf exposure by image analysis. Aust. J. Grape Wine Res. 2019, 25, 363–374. [Google Scholar] [CrossRef]
- Somogyi, E.; Lázár, J.; Baranyai, L.; Bodor-Pesti, P.; Nyitrainé Sárdy, D.Á. Outline analysis of the grapevine (Vitis vinifera L.) berry shape by elliptic Fourier descriptors. Vitis 2022, 61, 63–70. [Google Scholar]
- Bodor, P.; Somogyi, E.; Baranyai, L.; Lázár, J.; Bálo, B. Analysis of the grapevine (Vitis vinifera L.) berry shape by using elliptic Fourier descriptors. Agric. Eng. Sci. 2020, 16, 87–93. [Google Scholar] [CrossRef]
- OIV. OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; Office International de la Vigne et du Vin: Paris, France, 2009; p. 177. [Google Scholar]
- Bodor, P.; Baranyai, L.; Parrag, V.; Bisztray, G. Effect of row orientation and elevation on leaf morphology of grapevine (Vitis vinifera L.) cv Furmint. Prog. Agric. Eng. Sci. 2014, 10, 53–69. [Google Scholar]
- Poni, S.; Intrieri, C.; Magnanini, E. Seasonal growth and gas exchange of conventionally and minimally pruned Chardonnay canopies. Vitis 2000, 39, 13–18. [Google Scholar]
- Ruberti, C.; Barizza, E.; Bodner, M.; La Rocca, N.; De Michele, R.; Carimi, F.; Lo Schiavo, F.; Zottini, M. Mitochondria change dynamics and morphology during grapevine leaf senescence. PLoS ONE 2014, 9, e102012. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Klouvatou, A.; Vekkos, K.A.; Manetas, Y.; Karabourniotis, G. The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann. Bot. 2006, 98, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Ates, F.; Kaya, O. The relationship between iron and nitrogen concentrations based on Kjeldahl method and SPAD-502 readings in grapevine (Vitis vinifera L. cv.‘Sultana Seedless’). Erwerbs-Obstbau 2021, 63, 53–59. [Google Scholar] [CrossRef]
- Casanova-Gascón, J.; Martín-Ramos, P.; Martí-Dalmau, C.; Badía-Villas, D. Nutrients assimilation and chlorophyll contents for different grapevine varieties in calcareous soils in the Somontano DO (Spain). Beverages 2018, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Petit, A.N.; Vaillant, N.; Boulay, M.; Clément, C.; Fontaine, F. Alteration of photosynthesis in grapevines affected by esca. Phytopathology 2006, 96, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlousek, P. Evaluation of drought tolerance of new grapevine rootstock hybrids. J. Environ. Biol. 2011, 32, 543. [Google Scholar] [PubMed]
- Porro, D.; Dorigatti, C.; Stefanini, M.; Ceschini, A. Use of SPAD meter in diagnosis of nutritional status in apple and grapevine. Acta Hortic. 2001, 564, 243–252. [Google Scholar] [CrossRef]
- Zulini, L.; Rubinigg, M.; Zorer, R.; Bertamini, M. Effects of drought stress on chlorophyll fluorescence and photosynthetic pigments in grapevine leaves (Vitis vinifera cv. ‘White Riesling’). Int. Workshop Adv. Grapevine Wine Res. 2005, 754, 289–294. [Google Scholar]
- Kawashima, S.; Nakatani, M. An Algorithm for Estimating Chlorophyll Content in Leaves Using a Video Camera. Ann. Bot. 1998, 81, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Guendouz, A.; Bendada, H.; Benniou, R. A comparative study of chlorophyll content estimation in barley (Hordeum vulgare L.) genotypes based on RGB (Red, Green, Blue) image analysis. Agric. Sci. Dig. Res. J. 2022, 42, 68–71. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, X.; Gao, L.; Li, C.; Wang, L.; Zhao, G.; Jiang, Y. Estimation of chlorophyll content in apple leaves based on RGB model using digital camera. Acta Hortic. Sin. 2017, 44, 381–390. [Google Scholar]
- Özreçberoğlu, N.; Kahramanoğlu, İ. Mathematical models for the estimation of leaf chlorophyll content based on RGB colours of contact imaging with smartphones: A pomegranate example. Folia Hortic. 2020, 32, 57–67. [Google Scholar] [CrossRef]
- Sala, F.; Datcu, A.D.; Kolozsvári, A.G. RGB color parameters in the characterization of chlorophyll deficiency in leaves. Case study: Birch. Ann. West Univ. Timis. Ser. Biol. 2020, 23, 241–250. [Google Scholar]
- Sánchez-Sastre, L.F.; Alte da Veiga, N.M.S.; Ruiz-Potosme, N.M.; Carrión-Prieto, P.; Marcos-Robles, J.L.; Navas-Gracia, L.M.; Martín-Ramos, P. Assessment of RGB Vegetation Indices to Estimate Chlorophyll Content in Sugar Beet Leaves in the Final Cultivation Stage. AgriEngineering 2020, 2, 128–149. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; d’Andria, R.; Jacobsen, S.E. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Apogee Chlorophyll Meter Support. Available online: https://www.apogeeinstruments.com/chlorophyll-meter-support/#specifications (accessed on 1 February 2023).
- Chavarria, G.; Santos, H.P.D.; Castro, L.A.S.D.; Marodin, G.A.B.; Bergamaschi, H. Anatomy, chlorophyll content and photosynthetic potential in grapevine leaves under plastic cover. Rev. Bras. Frutic. 2012, 34, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Eitel, J.U.; Engels, M.; Zhu, J.; Ma, Y.; Liao, F.; Zheng, H.; Wang, X.; Yao, X.; Cheng, T.; et al. Improving Unmanned Aerial Vehicle (UAV) remote sensing of rice plant potassium accumulation by fusing spectral and textural information. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102592. [Google Scholar] [CrossRef]
- Fuentes, S.; Hernández-Montes, E.; Escalona, J.M.; Bota, J.; Viejo, C.G.; Poblete-Echeverría, C.; Tongson, E.; Medrano, H. Automated grapevine cultivar classification based on machine learning using leaf morpho-colorimetry, fractal dimension and near-infrared spectroscopy parameters. Comput. Electron. Agric. 2018, 151, 311–318. [Google Scholar] [CrossRef]
- Doğan, A.; Uyak, C. A different approach for grape leaf color. J. Agric. Fac. Gaziosmanpasa Univ. 2020, 37, 44–52. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodor-Pesti, P.; Taranyi, D.; Nyitrainé Sárdy, D.Á.; Le Phuong Nguyen, L.; Baranyai, L. Correlation of the Grapevine (Vitis vinifera L.) Leaf Chlorophyll Concentration with RGB Color Indices. Horticulturae 2023, 9, 899. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae9080899
Bodor-Pesti P, Taranyi D, Nyitrainé Sárdy DÁ, Le Phuong Nguyen L, Baranyai L. Correlation of the Grapevine (Vitis vinifera L.) Leaf Chlorophyll Concentration with RGB Color Indices. Horticulturae. 2023; 9(8):899. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae9080899
Chicago/Turabian StyleBodor-Pesti, Péter, Dóra Taranyi, Diána Ágnes Nyitrainé Sárdy, Lien Le Phuong Nguyen, and László Baranyai. 2023. "Correlation of the Grapevine (Vitis vinifera L.) Leaf Chlorophyll Concentration with RGB Color Indices" Horticulturae 9, no. 8: 899. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae9080899