Spontaneous Magnetization and Optical Activity in the Chiral Series {(L-proline)nV[Cr(CN)6]x} (0 < n < 3)
Abstract
:1. Introduction
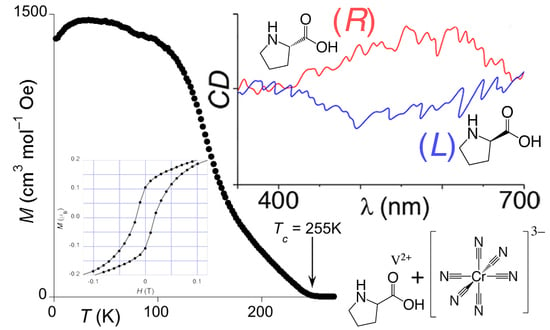
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, J.S. Magnetically ordered molecule-based materials. Chem. Soc. Rev. 2011, 40, 3266–3296. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Czarnecki, B.; Reczynski, M.; Arczynski, M. Multifunctionality in molecular magnetism. Sci. Prog. 2015, 98, 346–378. [Google Scholar] [CrossRef]
- Hozumi, T.; Hashimoto, K.; Ohkoshi, S. Electrochemical synthesis, crystal structure, and photomagnetic properties of a three-dimensional cyano-bridged copper-molybdenum complex. J. Am. Chem. Soc. 2005, 127, 3864–3869. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet. Nat. Chem. 2011, 3, 564–569. [Google Scholar] [CrossRef]
- Aguilà, D.; Prado, Y.; Koumousi, E.S.; Mathonière, C.; Clérac, R. Clerac, Switchable Fe/Co Prussian blue networks and molecular analogues. Chem. Soc. Rev. 2016, 45, 203–224. [Google Scholar] [CrossRef] [Green Version]
- Magott, M.; Stefanczyk, O.; Sieklucka, B.; Pinkowicz, D. Octacyanidotungstate(IV) coordination chains demonstrate a light-induced excited spin state trapping behavior and magnetic exchange photoswitching. Angew. Chem. Int. Ed. 2017, 56, 13283–13287. [Google Scholar] [CrossRef]
- Darago, L.E.; Aubrey, M.L.; Yu, C.J.; Gonzalez, M.I.; Long, J.R. Electronic conductivity, ferrimagnetic ordering and reductive insertion mediated by organic mixed-valence in a ferric semiquinoid metal-organic framework. J. Am. Chem. Soc. 2015, 137, 15703–15711. [Google Scholar] [CrossRef]
- Day, P.; Kurmoo, M. Molecular magnetic semiconductors, metals and superconductors: BEDT-TTF salts with magnetic anions. J. Mater. Chem. 1997, 7, 1291–1295. [Google Scholar] [CrossRef]
- Ojima, E.; Fujiwara, H.; Kato, K.; Kobayashi, H.; Tanaka, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetic organic metal exhibiting superconducting transition, κ-(BETS)2FeBr4. J. Am. Chem. Soc. 1999, 121, 5581–5582. [Google Scholar] [CrossRef]
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Mu, K.; Shan, H.; Guo, Y.; Wu, J.; Su, Y.; Wu, Q.; Sun, Z.; Zhao, A.; et al. Molecule-confined engineering towards superconductivity and ferromagneticm in two-dimensional superlattice. J. Am. Chem. Soc. 2017, 139, 16398–16404. [Google Scholar] [CrossRef]
- Hedley, L.; Robertson, N.; Johansson, J.O. Electrochromic thin films of the V-Cr Prussian blue analogue molecular magnet. Electrochim. Acta 2017, 236, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Beghidja, A.; Rogez, G.; Rabu, P.; Welter, R.; Drillon, M. An approach to chiral magnets using α-hydroxycarboxylates. J. Mater. Chem. 2006, 16, 2715–2728. [Google Scholar] [CrossRef]
- Numata, Y.; Inoue, K.; Baranoc, N.; Kurmoo, M.; Kikuchi, K. Field-induced ferrimagnetic state in a molecule-based magnet consisting of a CoII ion and a chiral triplet bis(nitroxide) radical. J. Am. Chem. Soc. 2007, 129, 9902–9909. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R.; Coronado, E.; Goddard, P.A.; Singleton, J.; Coldea, A.I.; Wallis, J.D.; Coles, S.J.; Alberola, A. A chiral ferromagnetic molecular metal. J. Am. Chem. Soc. 2010, 132, 9271–9273. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nitek, W.; Rams, M.; Majcher, A.M.; Nuida, T.; Ohkoshi, S.; Sieklucka, B. Multifunctional magentic molecular {[MnII(urea)2(H2O)]2[NbIV(CN)8]}n system: Magnetization-induced SHG in the chiral polymorph. Chem. Mater. 2011, 23, 21–31. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Takano, S.; Imoto, K.; Yoshikiyo, M.; Namai, A.; Tokoro, H. 90-degree optical switching of output second-harmonic light in chiral photomagnet. Nat. Photonics 2014, 8, 65–71. [Google Scholar] [CrossRef]
- Barron, L.D.; Vrbancich, J. Magneto-chiral birefringence and dichroism. Mol. Phys. 1984, 51, 715–730. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Raupach, E. Observation of magnetochiral dichroism. Nature 1997, 390, 493–494. [Google Scholar] [CrossRef]
- Mallah, T.; Thiébaut, S.; Verdaguer, M.; Veillet, P. High-TC molecular-based magnets: Ferrimagnetic mixed-valence chromium(III)-chromium(II) cyanides with TC at 240 and 190 K. Science 1993, 262, 1554–1558. [Google Scholar] [CrossRef]
- Buschmann, W.E.; Paulson, S.C.; Wynn, C.M.; Girtu, M.A.; Epstein, A.J.; White, H.S.; Miller, J.S. Magnetic field induced reversed (negative) magnetization for electrochemically deposited TC = 260 K oxidized films of chromium cyanide magnets. Adv. Mater. 1997, 9, 645–647. [Google Scholar] [CrossRef]
- Johansson, J.O.; Kim, J.W.; Allwright, E.; Rogers, D.M.; Robertson, N.; Bigot, J.Y. Directly probing spin dynamics in a molecular magnet with femtosecond time-resolution. Chem. Sci. 2016, 7, 7061–7067. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, W.; Imoto, K.; Tsunobuchi, Y.; Ohkoshi, S. Vanadium octacyanoniobate-based magnet with a Curie temperature of 138 K. Inorg. Chem. 2009, 48, 4604–4606. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodríguez-Fortea, A.; Álvarez, S.; Verdaguer, M. Is it possible to get high TC magnets with Prussian blue analogues? A theoretical prospect. Chem. Eur. J. 2005, 11, 2135–2144. [Google Scholar] [CrossRef]
- Ferlay, S.T.; Mallah, T.; Ouahès, R.; Veillet, P.; Verdaguer, M. A room-temperature organometallic magnet based on Prussian blue. Nature 1995, 378, 701–703. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Buschmann, W.E.; Zhang, J.; Manson, J.L.; Miller, J.S. Enhancement of the magnetic ordering temperature and air stability of a mixed valent vanadium hexacyanochromate(III) magnet to 99 °C (372 K). Adv. Mater. 1999, 11, 914–918. [Google Scholar] [CrossRef]
- Holmes, S.M.; Girolami, G.S. Sol-gel synthesis of KVII[CrIII(CN)6]∙2H2O: A crystalline molecule-based magnet with a magnetic ordering temperature above 100 °C. J. Am. Chem. Soc. 1999, 121, 5593–5594. [Google Scholar] [CrossRef]
- Ferlay, S.; Mallah, T.; Ouahès, R.; Veillet, P.; Verdaguer, M. A chromium-vanadyl ferrimagnetic molecule-based magnet: Structure, magnetism, and orbital interpretation. Inorg. Chem. 1999, 38, 229–234. [Google Scholar] [CrossRef]
- Coronado, E.; Gimenez-Saiz, C.; Martinez-Agudo, J.M.; Nuez, A.; Romero, F.M.; Stoecklie-Evans, H. Design of chiral magnets: Cyanide-bridged bimetallic assemblies based on cyclohexane-1,2-diamine. Polyhedron 2003, 22, 2435–2440. [Google Scholar] [CrossRef]
- Coronado, E.; Gomez-Garcia, C.J.; Nuez, A.; Romero, F.M.; Waerenborgh, J.C. Synthesis, chirality, and magnetic properties of bimetallic cyanide-bridged two-dimensional ferromagnets. Chem. Mater. 2006, 18, 2670–2681. [Google Scholar] [CrossRef]
- Millon, J.; Daniel, M.C.; Kaiba, A.; Guionneau, P.; Brandes, S.; Sutter, J.P. Nanoporous magnets of chiral and racemic [{Mn(HL)}2Mn{Mo(CN)7}2] with switchable ordering temperatures (TC = 85 K ↔ 106 K) driven by H2O sorption (L = N,N-dimethylalaninol). J. Am. Chem. Soc. 2007, 129, 13872–13878. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gomez-Garcia, C.J.; Nuez, A.; Romero, F.M.; Rusanov, E.; Stoeckli-Evans, H. Ferromagnetism and chirality in two-dimensional cyanide-bridged bimetallic compounds. Inorg. Chem. 2002, 41, 4615–4617. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, W.; Kitagawa, S.; Ohba, M. Chiral cyanide-bridged MnIIMnIII ferrimagnets, [MnII(HL)(H2O)][MnIII(CN)6]∙2H2O (L = S- or R-1,2-diaminopropane): Syntheses, structures, and magnetic behaviors. J. Am. Chem. Soc. 2007, 129, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Train, C.; Gruselle, M.; Verdaguer, M. The fruitful introduction of chirality and control of absolute configurations in molecular magnets. Chem. Soc. Rev. 2011, 40, 3297–3312. [Google Scholar] [CrossRef]
- Garde, R.; Herrera, J.M.; Villain, F.; Verdaguer, M. Molecule-based magnets with TC above room temperature: Improved synthesis of vanadium-chromium Prussian blue analogues with inserted alkali cations. Inorg. Chim. Acta 2008, 361, 3597–3602. [Google Scholar] [CrossRef]
- Dujardin, E.; Ferlay, S.; Phan, X.; Desplanches, C.; Cartier dit Moulin, C.; Sainctavit, P.; Baudelet, F.; Dartyge, E.; Veillet, P.; Verdaguer, M. Synthesis and magnetization of new room-temperature molecule-based magnets: Effect of stoichiometry on local magnetic structure by X-ray magnetic circular dichroism. J. Am. Chem. Soc. 1998, 120, 11347–11352. [Google Scholar] [CrossRef]
- Garde, R.; Villain, F.; Verdaguer, M. Molecule-based room-temperature magnets: Catalytic role of V(III) in the synthesis of vanadium–chromium Prussian blue analogues. J. Am. Chem. Soc. 2002, 124, 10531–10538. [Google Scholar] [CrossRef]
- Train, C.; Gheorghe, R.; Krstic, V.; Chamoreau, L.-M.; Ovanesyan, N.S.; Rikken, G.L.J.A.; Gruselle, M.; Verdaguer, M. Strong magneto-chiral dichroism in enantiopure chiral ferormagnets. Nat. Mater. 2008, 7, 729–734. [Google Scholar] [CrossRef]
- Ceolín, M.; Goberna-Ferrón, S.; Galan-Mascaros, J.R. Strong hard X-ray magnetochiral dichroism in paramagnetic enantiopure molecules. Adv. Mater. 2012, 24, 3120–3123. [Google Scholar] [CrossRef]
- Sessoli, R.; Boulon, M.-E.; Caneschi, A.; Mannini, M.; Poggini, L.; Wilhem, F.; Rogalev, A. Strong magneto-chiral dichroism in a paramagnetic molecular helix observed by hard X-rays. Nat. Phys. 2015, 11, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Atzori, M.; Breslavetz, I.; Paillot, K.; Inoue, K.; Rikken, G.L.J.A.; Train, C. A chiral Prussian blue analogue pushes magneto-chiral dichroism limits. J. Am. Chem. Soc. 2019, 141, 20022–20025. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-García, B.; Galan-Mascaros, J.R. Spontaneous Magnetization and Optical Activity in the Chiral Series {(L-proline)nV[Cr(CN)6]x} (0 < n < 3). Magnetochemistry 2020, 6, 12. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry6010012
Rodríguez-García B, Galan-Mascaros JR. Spontaneous Magnetization and Optical Activity in the Chiral Series {(L-proline)nV[Cr(CN)6]x} (0 < n < 3). Magnetochemistry. 2020; 6(1):12. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry6010012
Chicago/Turabian StyleRodríguez-García, Bárbara, and Jose Ramon Galan-Mascaros. 2020. "Spontaneous Magnetization and Optical Activity in the Chiral Series {(L-proline)nV[Cr(CN)6]x} (0 < n < 3)" Magnetochemistry 6, no. 1: 12. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry6010012