A Contemporary Exploration of Traditional Indian Snake Envenomation Therapies
Abstract
:1. Introduction
2. Materials and Methods
- The article mentions the use of the whole plant, its part or extracted component as a therapy to treat snake envenomation and has ethnobotanical or folklore evidence.
- The article describes pharmacological research related to the neutralization of snake venom or its component by the specified plant, its part or extracted component.
- Multiple articles that cross-refer the same source of traditional use or pharmacological research without additional or unique particulars were not included.
3. The Traditional Therapies for Snake Envenomation
3.1. Large Formulations for Snake Envenomation
3.2. Therapeutic Anomalies
3.3. Toxicity of Plants
4. Snake Envenomation Prophylaxis
5. Snake Repellents
6. Routes of Administration
6.1. Oral
6.2. Topical
6.3. Ocular
6.4. Nasal
7. Contemporary Exploration of Plants as Antidotes for Snake Venom
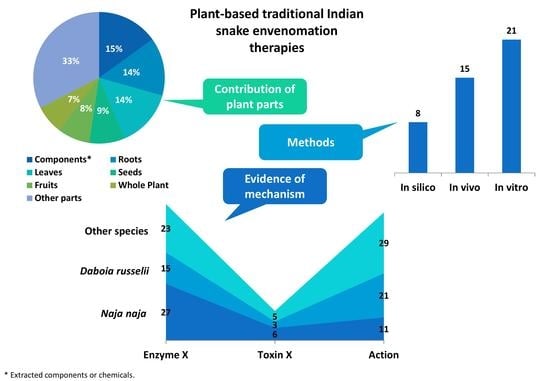
8. Plant Parts
9. Snake Species with Modern Evidence of Venom Neutralization
9.1. Pharmacological Action
9.2. Enzyme Inhibition
9.3. Toxin Inhibition
10. Plant Species with Modern Evidence of Snake Venom Neutralization
11. Opportunities with a Few Additional Plant Species
12. The Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, A.T. Overview of Snake Venom Chemistry. In Natural Toxins II; Advances in Experimental Medicine and Biology; Singh, B.R., Tu, A.T., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 37–62. [Google Scholar] [CrossRef]
- Iwanaga, S.; Suzuki, T. Enzymes in Snake Venom. In Snake Venoms; Lee, C.-Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 61–158. [Google Scholar] [CrossRef]
- WHO. Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 5 September 2021).
- Snake-bite envenoming: A priority neglected tropical disease. Lancet 2017, 370, P2. [CrossRef] [Green Version]
- Williams, D.J.; Abul Faiz, M.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Wen Fan, H.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punde, D.P. Management of snake-bite in rural Maharashtra: A 10-year experience. Natl. Med. J. India 2005, 18, 71. [Google Scholar] [PubMed]
- Mehta, S.R.; Sashindran, V.K. Clinical Features and Management of Snake Bite. Med. J. Armed Forces India 2002, 58, 247. [Google Scholar] [CrossRef] [Green Version]
- Meenatchisundaram, S.; Michael, A. Snake bite and therapeutic measures: Indian scenario. Indian J. Sci. Technol. 2009, 2, 69–73. [Google Scholar] [CrossRef]
- Pade, S.D.; Patil, P.B.; Gadre, D.V.; Padhye-Gurjar, A.B. Aushadhi Baad; Rajesh Prakashan: Pune, India, 2010; Volume 1–3. [Google Scholar]
- Martz, W. Plants with a reputation against snakebite. Toxicon 1992, 30, 1131–1142. [Google Scholar] [CrossRef]
- Mors, W.B.; do Nascimento, M.C.; Ruppelt Pereira, B.M.; Pereira, N.A. Plant natural products active against snake bite—The molecular approach. Phytochemistry 2000, 55, 627–642. [Google Scholar] [CrossRef]
- Houghton, P.J.; Osibogun, I.M. Flowering plants used against snakebite. J. Ethnopharmacol. 1993, 39, 1–29. [Google Scholar] [CrossRef]
- Coe, F.G.; Anderson, G.J. Snakebite ethnopharmacopoeia of eastern Nicaragua. J. Ethnopharmacol. 2005, 96, 303–323. [Google Scholar] [CrossRef]
- Soares, A.M.; Ticli, F.K.; Marcussi, S.; Lourenco, M.V.; Januario, A.H.; Sampaio, S.V.; Giglio, J.R.; Lomonte, B.; Pereira, P.S. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005, 12, 2625–2641. [Google Scholar] [CrossRef]
- Mebs, D. Notes on the traditional use of plants to treat snake bite in northern Papua New Guinea. Toxicon 2000, 38, 299–302. [Google Scholar] [CrossRef]
- Kanojia, A.; Chaudhari, K.S.; Gothecha, V.K. Medicinal plants active against snake envenomation. Int. J. Res. Ayurveda Pharm. 2012, 3, 363–366. [Google Scholar]
- Samy, R.P.; Thwin, M.M.; Gopalakrishnakone, P.; Ignacimuthu, S. Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J. Ethnopharmacol. 2008, 115, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Khyade, M.S.; Takate, Y.A.; Divekar, M.V. Plants Used as an Antidote Against Snakebite in Akole Taluka of Ahmednagar District (MS), India. J. Nat. Remedies 2011, 11, 182–192. [Google Scholar]
- Kadel, C.; Jain, A.K. Folklore claims on snakebite among some tribal communities of Central India. Indian J. Tradit. Knowl. 2008, 7, 296–299. [Google Scholar]
- Pade, S.D. Vanaushadhi Gunadarsha; Shri Gajanan Book Depot: Pune, India, 1893; Volume 1–7. [Google Scholar]
- Sathe, K.N. Gharguti Aushadhe, 16th ed.; Shailaja Anil Sathe: Mumbai, India, 2003. [Google Scholar]
- Timmins, R.J.; Duckworth, J.W. Moschus cupreus. In The IUCN Red List Threatened Species 2015; 2015; p. e.T136750A61979453. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Dickers, K.J.; Rice, P.; Griffiths, G.D.; Vale, J.A. Ricin Poisoning. Toxicol. Rev. 2003, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.F.; Mota, É.F.; Silva, A.C.M.; Tomé, A.R.; Silva, M.Z.; de Brito, D.; Porfírio, C.T.; Oliveira, A.C.; Lima-Filho, J.V.; Ramos, M.V. Latex proteins from Calotropis procera: Toxicity and immunological tolerance revisited. Chem. Biol. Interact. 2017, 274, 138–149. [Google Scholar] [CrossRef]
- Anger, E.E.; Yu, F.; Li, J. Aristolochic acid-induced nephrotoxicity: Molecular mechanisms and potential protective approaches. Int. J. Mol. Sci. 2020, 21, 1157. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.; Lokesh, C.R.; Sahu, S.; Gupta, N. Luffa echinata: Healer plant or potential killer? J. Postgrad. Med. 2014, 60, 72. [Google Scholar] [CrossRef]
- Stirpe, F.; Pession-Brizzi, A.; Lorenzoni, E.; Strocchi, P.; Montanaro, L.; Sperti, S. Studies on the proteins from the seeds of Croton tiglium and of Jatropha curcas. Toxic properties and inhibition of protein synthesis in vitro. Biochem. J. 1976, 156, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Barth, A.; Müller, D.; Dürrling, K. In vitro investigation of a standardized dried extract of Citrullus colocynthis on liver toxicity in adult rats. Exp. Toxicol. Pathol. 2002, 54, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Naidu, M.T.; Babu, N.C.; Venkaiah, M. Ethnic remedies against snake bite from Kotia hills of Vizianagaram district, Andhra Pradesh, India. Indian J. Nat. Prod. Resour. 2013, 4, 194–196. [Google Scholar]
- Roy, K. India’s Historic Battles from Alexander the Great to Kargil; Permanent Black: Delhi, India, 2004; p. 201. [Google Scholar]
- Penzer, N.M.; Bhaṭṭa, S. Poison Damsels: Folklore of the World; Arno Press: New York, NY, USA, 1980; p. 319. [Google Scholar]
- Rao, C.V.; Verma, A.R.; Gupta, P.K.; Madhavan, V. Anti-inflammatory and anti-nociceptive activities of Fumaria indica whole plant extract in experimental animals. Acta Pharm. 2007, 57, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Alm, T. Dyvelsdrek Ferula assa-foetida i folketradisjonen i Norge-med noen klassiske sidesprang. Blyttia 2004, 62, 14–48. [Google Scholar]
- Wadhwani, B.D.; Mali, D.; Vyas, P.; Nair, R.; Khandelwal, P. A review on phytochemical constituents and pharmacological potential of Calotropis procera. RSC Adv. 2021, 11, 35854–35878. [Google Scholar] [CrossRef]
- Timmins, R.; Kawanishi, K.; Giman, B.; Lynam, A.; Chan, B.; Steinmetz, R.; Sagar Baral, H.; Samba Kumar, N. Rusa unicolor. In IUCN Red List Threatened Species 2015; (Errata Version Published in 2015); 2015; p. e.T41790A85628124. [Google Scholar] [CrossRef]
- Widyowati, R.; Suciati, S.; Haryadi, D.M.; Chang, H.-I.; Suryawan, I.N.; Utama, A.W. The effect of Rusa unicolor antler deer extracts from East Kalimantan in bone turnover cell models. Turk. J. Pharm. Sci. 2020, 17, 440–445. [Google Scholar] [CrossRef]
- Savrikar, S.S.; Ravishankar, B. Bhaishajya Kalpanaa—The Ayurvedic pharmaceutics—An overview. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Reid, H.A.; Theakston, R.D.G. The management of snake bite. Bull. World Health Organ. 1983, 61, 885–895. [Google Scholar]
- Patil, V.M.; Das, S.; Balasubramanian, K. Quantum chemical and docking insights into bioavailability enhancement of curcumin by piperine in pepper. J. Phys. Chem. A 2016, 120, 3643–3653. [Google Scholar] [CrossRef]
- Khajuria, A.; Zutshi, U.; Bedi, K. Permeability characteristics of piperine on oral absorption-an active alkaloid from peppers and a bioavailability enhancer. Indian J. Exp. Biol. 1998, 36, 46–50. [Google Scholar] [PubMed]
- Shenoy, P.A.; Nipate, S.S.; Sonpetkar, J.M.; Salvi, N.C.; Waghmare, A.B.; Chaudhari, P.D. Anti-snake venom activities of ethanolic extract of fruits of Piper longum L. (Piperaceae) against Russell’s viper venom: Characterization of piperine as active principle. J. Ethnopharmacol. 2013, 147, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.A.; Nipate, S.S.; Sonpetkar, J.M.; Salvi, N.C.; Waghmare, A.B.; Chaudhari, P.D. Production of high titre antibody response against Russell’s viper venom in mice immunized with ethanolic extract of fruits of Piper longum L. (Piperaceae) and piperine. Phytomedicine 2014, 21, 159–163. [Google Scholar] [CrossRef]
- Ghag-Sawant, M.; More, T.V.; Samant, L.S.; Chowdhary, A.S. Study of neutralization of enzymatic activity of Daboia russelii venom by various plant extracts and their combinations using in vitro methods. Int. J. Pharm. Sci. Res. 2016, 7, 2531–2536. [Google Scholar] [CrossRef]
- Dave, B.J.; Trivedi, A.H.; Adhvatyu, S.G. Role of areca nut consumption in the cause of oral cancers. A cytogenetic assessment. Cancer 1992, 70, 1017–1023. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Trivedy, C.; Peters, T.J. Areca nut use: An independent risk factor for oral cancer: The health problem is under-recognised. Br. Med. J. 2002, 324, 799–800. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 85, 334. [Google Scholar]
- Merchant, A.; Husain, S.S.M.; Hosain, M.; Fikree, F.F.; Pitiphat, W.; Siddiqui, A.R.; Hayder, S.J.; Haider, S.M.; Ikram, M.; Chuang, S.K.; et al. Paan without tobacco: An independent risk factor for oral cancer. Int. J. Cancer 2000, 86, 128–131. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Dobos, G.J.; Rampp, T. Clinical significance of leech therapy in Indian medicine. J. Evid. Based Complement. Altern. Med. 2013, 18, 152–158. [Google Scholar] [CrossRef]
- Adams, S.L. The medicinal leech: A page from the annelids of internal medicine. Ann. Intern. Med. 1988, 109, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, D.; Aurich, M.; Pasalar, M.; Rampp, T. Medicinal leech therapy in venous congestion and various ulcer forms: Perspectives of Western, Persian and Indian medicine. J. Tradit. Complement. Med. 2020, 10, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Snake venom components affecting blood coagulation and the vascular system: Structural similarities and marked diversity. Curr. Pharm. Des. 2007, 13, 2872–2886. [Google Scholar] [CrossRef] [PubMed]
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug. Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kublik, H.; Vidgren, M.T. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Ghori, M.U.; Mahdi, M.H.; Smith, A.M.; Conway, B.R. Nasal drug delivery systems: An overview. Am. J. Pharmacol. Sci. 2015, 3, 110–119. [Google Scholar] [CrossRef]
- Dorrigiv, M.; Zareiyan, A.; Hosseinzadeh, H. Garlic (Allium sativum) as an antidote or a protective agent against natural or chemical toxicities: A comprehensive update review. Phytother. Res. 2020, 34, 1770–1797. [Google Scholar] [CrossRef]
- Nencini, C.; Franchi, G.G.; Cavallo, F.; Micheli, L. Protective effect of Allium neapolitanum Cyr. versus Allium sativum L. on acute ethanol-induced oxidative stress in rat liver. J. Med. Food 2010, 13, 329–335. [Google Scholar] [CrossRef]
- Venugopal, P.V.; Venugopal, T.V. Antidermatophytic activity of garlic (Allium sativum) in vitro. Int. J. Dermatol. 1995, 34, 278–279. [Google Scholar] [CrossRef]
- Asante-Kwatia, E.; Mensah, A.Y.; Fobi, E. An ethnobotanical study on medicinal plants used as antidote for snakebite and as snake repellent in the Ejisu-Juabeng District of Ghana. Res. J. Pharmacogn. 2021, 8, 53–62. [Google Scholar] [CrossRef]
- Asad, M.H.H.B.; Durr-E-Sabih; Yaqab, T.; Murtaza, G.; Hussain, M.S.; Hussain, M.S.; Nasir, M.T.; Azhar, S.; Khan, S.A.; Hussain, I. Phospholipases A2: Enzymatic assay for snake venom (Naja naja karachiensis) with their neutralization by medicinal plants of Pakistan. Acta Pol. Pharm. Drug Res. 2014, 71, 625–630. [Google Scholar]
- Asad, M.H.H.B.; Murtaza, G.; Ubaid, M.; Sajjad, A.; Mehmood, R.; Mahmood, Q.; Ansari, M.M.; Karim, S.; Mehmood, Z.; Hussain, I. Naja naja karachiensis envenomation: Biochemical parameters for cardiac, liver, and renal damage along with their neutralization by medicinal plants. Biomed Res. Int. 2014, 2014, 970540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asad, M.H.H.B.; Razi, M.T.; Najamus-Saqib, Q.; Nasim, S.J.; Murtaza, G.; Hussain, I. Anti-venom potential of Pakistani medicinal plants: Inhibition of anticoagulation activity of Naja naja karachiensis toxin. Curr. Sci. 2013, 105, 1419–1424. [Google Scholar]
- Rahmy, T.R.; Hemmaid, K.Z. Prophylactic action of garlic on the histological and histochemical patterns of hepatic and gastric tissues in rats injected with a snake venom. J. Nat. Toxins 2001, 10, 137–165. [Google Scholar] [PubMed]
- Kuriakose, B.B.; Aleykutty, N.A.; Nitha, B. Evaluation of venom neutralising capacity of Indian medicinal plants by in vitro methods. Asian J. Pharm. Health Sci. 2012, 2, 552–554. [Google Scholar]
- Arif, M.; Rahman, M.A.; Imran, M.; Khalid, M.; Khushtar, M. An insight of Spondias mangifera willd: An underutilized medicinal plant with immense nutraceutical and therapeutic potentials. Int. J. Res. Pharm. Sci. 2015, 6, 100–109. [Google Scholar]
- Bahrami, G.; Soltani, R.; Sajjadi, S.-E.; Kanani, M.-R.; Naderi, R.; Ghiasvand, N.; Shokoohinia, Y. Essential oil composition of Ferula assa-foetida L. fruits from Western Iran. J. Rep. Pharm. Sci. 2013, 2, 90–97. [Google Scholar]
- Lenin; Rao, M.R.K.; Prabhu, K.; Bindu; Elizabeth, R.A.A.; Dinakar, S. The study of antioxidant activities of an Ayurvedic medicine Ayaskriti. Pharm. Lett. 2016, 8, 203–211. [Google Scholar]
- Sharma, R.; Thakur, G.S.; Sanodiya, B.S.; Savita, A.; Pandey, M.; Sharma, A.; Bisen, P.S. Therapeutic potential of Calotropis procera: A giant milkweed. IOSR J. Pharm. Biol. Sci. 2012, 4, 42–57. [Google Scholar] [CrossRef]
- Gomes, A.; Das, R.; Sarkhel, S.; Mishra, R.; Mukherjee, S.; Bhattacharya, S.; Gomes, A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010, 48, 865–878. [Google Scholar]
- Murti, Y.; Yogi, B.; Pathak, D. Pharmacognostic standardization of leaves of Calotropis procera (Ait.) R. Br.(Asclepiadaceae). Int. J. Ayurveda Res. 2010, 1, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Poonam; Punia, G. A review on varieties of Arka—Calotropis procera (Aiton) Dryand. and Calotropis gigantea (L.) Dryand. Glob. J. Res. Med. Plant Indig. Med. 2013, 2, 392–400. [Google Scholar]
- Alagesaboopathi, C. Ethnomedicinal plants and their utilization by villagers in Kumaragiri hills of Salem district of Tamilnadu, India. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kumari, A.; Sharma, M. Comparative GC-MS analysis of bioactive compounds in methanolic extract of Calotropis gigantea (L.) WT Aiton leaf and latex. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1823–1827. [Google Scholar]
- Shwetha, V.; Veena, S.M.; Govindappa, M.; Zameer, F.; Francois, N.N.; More, S.S. In vitro neutralization of Naja naja venom enzymes by folk medicinal plant extracts. J. Biol. Act. Prod. Nat. 2019, 9, 278–288. [Google Scholar] [CrossRef]
- Aslam, N.; Fatima, S.; Khalid, S.; Hussain, S.; Qayum, M.; Afzal, K.; Asad, M.H.H.B. Anti-5′-Nucleotidases (5′-ND) and Acetylcholinesterase (AChE) Activities of Medicinal Plants to Combat Echis carinatus Venom-Induced Toxicities. Biomed Res. Int. 2021, 2021, 6631042. [Google Scholar] [CrossRef] [PubMed]
- Kaladhar, D.S.V.G.K.; Duddukuri, G.R.; Ramesh, K.; Varahalarao Vadlapudi, V.; Yarla, N.S. In vitro protease Inhibition, Modulation of PLA2 Activity and Protein Interaction Studies of Calotropis gigantea. J. Clin. Cell. Immunol. 2013, 4, 1000165. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, V.; Menon, A.R.; Devaranavadagi, B. Identification of Daboia russelii snake venom Phospholipase A2 [PLA2] inhibitors present in methanolic root extract of Calotropis gigantea. Int. J. Pharm. Res. 2020, 178. [Google Scholar] [CrossRef]
- Sani, I.; Bello, F.; Fakai, I.M.; Abdulhamid, A. Evaluation of antisnake venom activities of some medicinal plants using albino rats. Sch. Int. J. Tradit. Complement. Med. 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Bhadane, B.S.; Patil, M.P.; Maheshwari, V.L.; Patil, R.H. Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: A review. Phytother. Res. 2018, 32, 1181–1210. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Palanivel, V.; Thangavelu, S.; Paramasivam, R.; Muthupandian, S. Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS ONE 2021, 16, e0256748. [Google Scholar] [CrossRef]
- Shamim, S.; Ahmad, S.I. Pharmacodynamic study on acute hypotensive activities of Carissa carandas extract in normal rats. Pak. J. Pharm. Sci. 2012, 25, 577–582. [Google Scholar] [PubMed]
- Tayoub, G.; Sulaiman, H.; Alorfi, M. Analysis of Oleandrin in Oleander extract (Nerium oleander) by HPLC. J. Nat. Prod. 2014, 7, 73–78. [Google Scholar]
- Mookerjee, D. A Case of Poisoning by Sheth Kurrubbee, the White Oleander. Ind. Med. Gaz. 1866, 1, 258A. [Google Scholar] [PubMed]
- Sinha, S.N.; Biswas, K. A concise review on Nerium oleander L.—An important medicinal plant. Trop. Plant Res. 2016, 3, 408–412. [Google Scholar]
- Siddiqui, S.; Hafeez, F.; Begum, S.; Siddiqui, B.S. Isolation and structure of two cardiac glycosides from the leaves of Nerium oleander. Phytochemistry 1986, 26, 237–241. [Google Scholar] [CrossRef]
- Makhija, I.K.; Khamar, D. Anti-snake venom properties of medicinal plants. Pharm. Lett. 2010, 2, 399–411. [Google Scholar]
- BBRG, V.L.; Nagavardhanam, N.; Pradesh, A.; Rani, G. Diversity of medicinal flora in and around Kolleru lake. Int. J. Adv. Res. Ideas Innov. Technol. 2018, 4, 5–10. [Google Scholar]
- Rahmatullah, M.; Mollik, M.A.H.; Ali, M.; Abbas, M.F.B.; Jahan, R.; Chowdhury, M.H.; Seraj, S.; Miajee Zumeu, A.A.K.; Bashar, A.B.M.A.; Chowdhury, A.R. An ethnomedicinal survey of Vitbilia village in Sujanagar sub-district of Pabna district, Bangladesh. Am. Eurasian J. Sustain. Agric. 2010, 4, 302–308. [Google Scholar]
- Sandey, H.; Sharma, A. Study on ethnomedicinal plants of Achanakmar-Amarkantak Tiger reserve of Chhattisgarh. J. Sci. Lett. 2016, 1, 216–222. [Google Scholar]
- Monachino, J. Rauvolfia serpentina—Its history, botany and medical use. Econ. Bot. 1954, 8, 349–365. [Google Scholar] [CrossRef]
- Trivedi, M.P.; Kumari, R. Ethno-botanical and Germinational Aspects of Rauvolfia serpentina (L.) Benth. Ex Kurz. Our Nature 2011, 9, 176–178. [Google Scholar] [CrossRef] [Green Version]
- Panda, D.; Kumar, S.; Padhan, B.; Nayak, K. Phytochemical evaluation of ethnomedicinal plants used against snake bite by the tribal people of koraput, Odisha, India. Ann. Ayurvedic Med. 2020, 9, 12–21. [Google Scholar]
- Dey, A.; De, J.N. Ethnobotanical aspects of Rauvolfia serpentina (L). Benth. ex Kurz. in India, Nepal and Bangladesh. J. Med. Plants Res. 2011, 5, 144–150. [Google Scholar]
- Sivaraman, T.; Sreedevi, N.S.; Meenachisundharam, S.; Vadivelan, R. Neutralizing potential of Rauvolfia serpentina root extract against Naja naja venom. Braz. J. Pharm. Sci. 2020, 56, e18050. [Google Scholar] [CrossRef]
- Sreekumar, S.; Nisha, N.; Biju, C.; Krishnan, P. Identification of potential lead compounds against cobra venom in Rauvolfia serpentina (L.) Benth. Ex kurz through molecular docking. Int. J. Pharm. Res. Dev. 2014, 6, 32–43. [Google Scholar]
- Joshi, Y.N.; Sodal, I.; Kale, T.; Patange, S.; Gote, V. Identification of Potential Lead Compounds Against Snake Neurotoxin in Rauvolfia serpentina Through Molecular Docking. Int. J. Sci. Res. Sci. Technol. 2020, 7, 180–186. [Google Scholar] [CrossRef]
- Dey, A.; Hazra, A.K.; Mukherjee, A.; Nandy, S.; Pandey, D.K. Chemotaxonomy of the ethnic antidote Aristolochia indica for aristolochic acid content: Implications of anti-phospholipase activity and genotoxicity study. J. Ethnopharmacol. 2021, 266, 113416. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; León, F. Perspective on the therapeutics of anti-snake venom. Molecules 2019, 24, 3276. [Google Scholar] [CrossRef] [Green Version]
- Padhy, G.K. A Review of Aristolochia indica: Ethnomedicinal Uses, Phytochemistry, Pharmacological and Toxicological Effects. Curr. Tradit. Med. 2021, 7, 372–386. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Peshin, S.S. Do herbal medicines have potential for managing snake bite envenomation? Toxicol. Int. 2012, 19, 89. [Google Scholar] [CrossRef] [Green Version]
- Rajashekharan, S.; Pushpangadan, P.; Kumar, P.K.R.; Jawahar, C.R.; Nair, C.P.R.; Amma, L.S. Ethno medico botanical studies of cheriya arayan and valiya arayan (Aristolochia indica, Linn; Aristolochia tagala, Cham). Anc. Sci. Life 1989, IX, 99–106. [Google Scholar]
- Minu, V.; Harsh, V.; Ravikant, T.; Paridhi, J.; Noopur, S. Medicinal plants of Chhattisgarh with anti-snake venom property. Int. J. Curr. Pharm. Rev. Res. 2012, 3, 1–10. [Google Scholar]
- Dey, A.; De, J.N. Anti-snake venom botanicals used by the ethnic groups of Purulia District, West Bengal, India. J. Herbs Spices Med. Plants 2012, 18, 152–165. [Google Scholar] [CrossRef]
- Alam, M. Inhibition of toxic effects of viper and cobra venom by Indian medicinal plants. Pharmacol. Pharm. 2014, 5, 48216. [Google Scholar] [CrossRef] [Green Version]
- Modak, B.K.; Gorai, P.; Pandey, D.K.; Dey, A.; Malik, T. An evidence based efficacy and safety assessment of the ethnobiologicals against poisonous and non-poisonous bites used by the tribals of three westernmost districts of West Bengal, India: Anti-phospholipase A2 and genotoxic effects. PLoS ONE 2020, 15, e0242944. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bhattacharyya, D. Characterization of the aqueous extract of the root of Aristolochia indica: Evaluation of its traditional use as an antidote for snake bites. J. Ethnopharmacol. 2013, 145, 220–226. [Google Scholar] [CrossRef]
- Meenatchisundaram, S.; Parameswari, G.; Michael, A. Studies on antivenom activity of Andrographis paniculata and Aristolochia indica plant extracts against Daboia russelli venom by in vivo and in vitro methods. Indian J. Sci. Technol. 2009, 2, 76–79. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. Inhibition of Naja naja venom hyaluronidase: Role in the management of poisonous bite. Life Sci. 2006, 78, 1433–1440. [Google Scholar] [CrossRef]
- Vishwanath, B.S.; Rao, A.G.A.; Gowda, T.V. Interaction of phospholipase A2 from Vipera russelli venom with aristolochic acid: A circular dichroism study. Toxicon 1987, 25, 939–946. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Bera, I.; Chakraborty, S.; Ghoshal, N.; Bhattacharyya, D. Aristolochic acid and its derivatives as inhibitors of snake venom L-amino acid oxidase. Toxicon 2017, 138, 1–17. [Google Scholar] [CrossRef]
- Ashok, P.; Koti, B.C.; Thippeswamy, A.H.M.; Tikare, V.P.; Dabadi, P.; Viswanathaswamy, A.H.M. Evaluation of antiinflammatory activity of Centratherum anthelminticum (L.) Kuntze seed. Indian J. Pharm. Sci. 2010, 72, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.-M.; Rajakumar, G.; Lee, J.-H.; Kim, S.-H.; Thiruvengadam, M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata. Appl. Microbiol. Biotechnol. 2017, 101, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Pithayanukul, P.; Laovachirasuwan, S.; Bavovada, R.; Pakmanee, N.; Suttisri, R. Anti-venom potential of butanolic extract of Eclipta prostrata against Malayan pit viper venom. J. Ethnopharmacol. 2004, 90, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mors, W.B.; Do Nascimento, M.C.; Parente, J.; Da Silva, M.H.; Melo, P.A.; Suarez-Kurtz, G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae). Toxicon 1989, 27, 1003–1009. [Google Scholar] [CrossRef]
- Melo, P.A.; Do Nascimento, M.C.; Mors, W.B.; Suarez-Kurtz, G. Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata (Asteraceae) extracts and constituents. Toxicon 1994, 32, 595–603. [Google Scholar] [CrossRef]
- Pithayanukul, P.; Lapett, B.; Bavovada, R.; Pakmanee, N.; Suttisri, R. Inhibition of Proteolytic and Hemorrhagic Activities by Ethyl Acetate Extract of Eclipta prostrata. Against Malayan Pit Viper Venom. Pharm. Biol. 2007, 45, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Chopra, R.N.; Dikshit, B.B.; Chowhan, J.S. Berberine and Berberine-containing plants in pharmacology and therapeutics. Ind. Med. Gaz. 1932, 67, 194–197. [Google Scholar]
- Kamal, G.C.; Gururaj, C.H. A review on Bilwadi Agada and its indications. World J. Pharm. Pharm. Sci. 2021, 10, 338–347. [Google Scholar] [CrossRef]
- Giri, A.; Mundhe, S.; Shimpi, M.; Gujrathi, D.S. Herbal antidotes for the management of snake bite. World J. Pharm. Pharm. Sci. 2019, 9, 735–743. [Google Scholar] [CrossRef]
- Omara, T.; Kagoya, S.; Openy, A.; Omute, T.; Ssebulime, S.; Kiplagat, K.M.; Bongomin, O. Antivenin plants used for treatment of snakebites in Uganda: Ethnobotanical reports and pharmacological evidences. Trop. Med. Health 2020, 48, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Saran, P.L.; Choudhary, R.; Solanki, I.S.; Devi, G. Traditional medicaments through papaya in North eastern plains zone of India. Indian J. Tradit. Knowl. 2015, 14, 537–543. [Google Scholar]
- Hukkeri, V.I.; Joshi, M.P.; Deshpande, M.N.; Nagare, S.K.; Korgaonkar, A.M. Phyto-pharmacological review of Terminalia chebula Retz. Nat. Prod. Indian J. 2010, 6, 24–28. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; Blatter, E., Caius, J.F., Mhaskar, K.S., Eds.; Lalit Mohan Basu: Allahabad, India, 1935; Volume II. [Google Scholar]
- Kapoor, M.; Kaur, N.; Sharma, C.; Kaur, G.; Kaur, R.; Batra, K.; Rani, J. Citrullus colocynthis an Important Plant in Indian Traditional System of Medicine. Pharmacogn. Rev. 2020, 14, 22–27. [Google Scholar] [CrossRef]
- Meena, M.C.; Meena, R.K.; Patni, V. Ethnobotanical studies of Citrullus colocynthis (Linn.) Schrad.—An important threatened medicinal herb. J. Med. Plants 2014, 2, 15–22. [Google Scholar]
- Kumar, V.; Rathee, P.; Kohli, K.; Chaudhary, H.; Rathee, S. Phytochemical and biological potential of indrayan: An overview. Pharmacogn. Rev. 2009, 3, 193. [Google Scholar]
- Jabeen, S.; Al Mahruqi, Z.M.H.; Nadeem, F.; Khalid, T. Bitter Apple (Citrullus colocynthis)—A Review of a Wild Plant Growing from Asia to Africa with High Medicinal Potentials. Int. J. Chem. Biochem. Sci. 2017, 11, 65–70. [Google Scholar]
- Asad, M.H.H.B.; Razi, M.T.; Murtaza, G.; Azhar, S.; Khan, S.A.; Saqib, Q.N.U.; Hussain, I. Antihaemorrhagic potential of Citrullus colocynthis schrad (cucurbitaceae) against Naja naja karachiensis (Black Pakistan cobra) venom. J. Med. Plants Res. 2012, 6, 3455–3458. [Google Scholar] [CrossRef]
- Wagh, V.V.; Jain, A.K. Traditional herbal remedies among Bheel and Bhilala tribes of Jhabua district Madhya Pradesh. Int. J. Biol. Technol. 2010, 1, 20–24. [Google Scholar]
- Das, D.R.; Sachan, A.K.; Shuaib, M.; Imtiyaz, M. Phyto-pharmacology of Momordca dioica: A review. Asian J. Pharm. Res. Dev. 2016, 4, 1–6. [Google Scholar]
- Patel, M.G.; Ishnava, K.B. Momordica dioica Roxb. (spine gourd): Multiple shoot induction from nodal cultures and its antidiabetic activity. J. Med. Plants Stud. 2015, 3, 82–88. [Google Scholar]
- Pawar, S. Traditional phytotherapy for animal bite among the tribal’s in Jalgaon district Maharashtra. Life Sci. Leafl. 2012, 8, 40–43. [Google Scholar]
- Weerasinghe, M.G.W.K.; Dahanayake, N. Momordica dioica Roxb. (Spine Gourd)- An underutilized vegetable and medicinal plant in Sri Lanka. Int. J. Minor Fruits Med. Aromat. Plants 2021, 7, 100–104. [Google Scholar] [CrossRef]
- Rupachandra, S.; Selvam, M.P.; Muthukumaran, N.; Senthilkumar, S.; Vaidhyalingam, S.; Dharshene, K. In Vitro Assessment of Cytotoxic Activity of Bioactive Peptides from Momordica dioica and Solanum trilobatum against Human Colon Cancer Cells. Biomed. Pharmacol. J. 2021, 14, 1007–1018. [Google Scholar] [CrossRef]
- Lim, T.K. Dioscorea bulbifera. In Edible Medicinal and Non-Medicinal Plants; Springer: Amsterdam, The Netherlands, 2016; Volume 10, pp. 235–252. [Google Scholar] [CrossRef]
- Esha, P.; Padiya, R.; Acharya, R.; Chauhan, M. Pharmacognostical Evaluation of Croton roxburghii Balak. (Euphorbiaceae) Bark. Ayurpharm Int. J. Ayurveda Allied Sci. 2013, 2, 58–62. [Google Scholar]
- Panda, S.K.; Dutta, S.K.; Bastia, A.K. Antibacterial activity of Croton roxburghii Balak. against the enteric pathogens. J. Adv. Pharm. Technol. Res. 2010, 1, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Chatatikun, M.; Yamauchi, T.; Yamasaki, K.; Aiba, S.; Chiabchalard, A. Anti melanogenic effect of Croton roxburghii and Croton sublyratus leaves in α-MSH stimulated B16F10 cells. J. Tradit. Complement. Med. 2019, 9, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mandal, L.; Bose, S. Pharmacognostic Standardization and Quantitative Estimation of some Isolated Phytoconstituents from Croton oblongifolius Roxb. J. PharmSciTech 2011, 1, 10–15. [Google Scholar]
- Mahmoud Aboulthana, W.; Youssef, A.; El-Feky, A.M.; El-Sayed Ibrahim, N.; Seif, M.M.; Kamal Hassan, A. Evaluation of antioxidant efficiency of Croton tiglium L. seeds extracts after incorporating silver nanoparticles. Egypt. J. Chem. 2019, 62, 181–200. [Google Scholar] [CrossRef]
- Simm, P.L. The supposed remedies for snake-bites. J. Soc. Arts 1856, 5, 101. [Google Scholar]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.d.F. Medicinal plants for the treatment of local tissue damage induced by snake venoms: An overview from traditional use to pharmacological evidence. Evid. Based Complement. Altern. Med. 2017, 2017, 5748256. [Google Scholar] [CrossRef]
- Khan, K.H. Roles of Emblica officinalis in medicine—A review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- Alam, M.I.; Gomes, A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J. Ethnopharmacol. 2003, 86, 75–80. [Google Scholar] [CrossRef]
- Sarkhel, S.; Chakravarty, A.K.; Das, R.; Gomes, A.; Gomes, A. Snake venom neutralising factor from the root extract of Emblica officinalis Linn. Orient. Pharm. Exp. Med. 2011, 11, 25–33. [Google Scholar] [CrossRef]
- Garaniya, N.; Bapodra, A. Ethno botanical and Phytophrmacological potential of Abrus precatorius L.: A review. Asian Pac. J. Trop. Biomed. 2014, 4, S27–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wambebe, C.; Amosun, S.L. Some neuromuscular effects of the crude extracts of the leaves of Abrus precatorius. J. Ethnopharmacol. 1984, 11, 49–58. [Google Scholar] [CrossRef]
- Vaidya, S.M.; Singh, A.R.; Patel, V.G.; Khan, N.A.; Yewale, R.P.; Kale, D.M.K. A review on herbs against snake venom. J. Pharmacogn. Phytochem. 2018, 7, 5–9. [Google Scholar] [CrossRef]
- Bhakta, S.; Das, S.K. The medicinal values of Abrus precatorius: A review study. J. Adv. Biotechnol. Exp. Ther. 2020, 3, 84–91. [Google Scholar] [CrossRef]
- Bhatia, M.; Siddiqui, N.; Gupta, S. Abrus precatorius (L.): An evaluation of traditional herb. J. Pharm. Res. 2013, 3, 3295–3315. [Google Scholar]
- Sajon, S.R.; Sana, S.; Rana, S. Anti-venoms for snake bite: A synthetic and traditional drugs review. J. Pharmacogn. Phytochem. 2017, 6, 190–197. [Google Scholar]
- Burli, D.A.; Khade, A.B. A comprehensive review on Butea monosperma (Lam.) Kuntze. Pharmacogn. Rev. 2007, 1, 333–337. [Google Scholar]
- Patil, M.V.; Pawar, S.; Patil, D.A. Ethnobotany of Butea monosperma (Lam.) Kuntze in North Maharashtra, India. Nat. Prod. Radiance 2006, 5, 323–325. [Google Scholar]
- Yadav, R.S.; Sharma, S.; Pasi, A.K.; Patel, S. Butea monosperma (PALASH): Plant Review with Their Phytoconstituents and Pharmacological applications. IOSR J. Pharm. Biol. Sci. 2020, 15, 18–23. [Google Scholar] [CrossRef]
- Firdaus, R.; Mazumder, A. Review on Butea monosperma. Int. J. Res. Pharm. Chem. 2012, 2, 1035–1039. [Google Scholar]
- Tarannum, S.; Mohamed, R.; Vishwanath, B.S. Inhibition of testicular and Vipera russelli snake venom hyaluronidase activity by Butea monosperma (Lam) Kuntze stem bark. Nat. Prod. Res. 2012, 26, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.M.; Patel, B.R. Phyto Pharmacological Perspective of Yashtimadhu (Glycyrrhiza glabra Linn.) A Review. Int. J. Pharm. Biol. Sci. Arch. 2013, 4, 833–841. [Google Scholar]
- Assafim, M.; Ferreira, M.S.; Frattani, F.S.; Guimarães, J.A.; Monteiro, R.Q.; Zingali, R.B. Counteracting effect of glycyrrhizin on the hemostatic abnormalities induced by Bothrops jararaca snake venom. Br. J. Pharmacol. 2006, 148, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Avinash, K.O.; Pradeep, S.; Shivamallu, C.; Gopenath, T.S.; Kumar, M.N.K.; Kanthesh, B.M. In silico screened flavanoids of Glycyrrhiza glabra Inhibit cPLA2 and sPLA2 in LPS stimulated macrophages. Bull. Environ. Pharmacol. Life Sci. 2021, 10, 14–24. [Google Scholar]
- Jitin, R.; Singh, S.P.; Naz, A. An ethnomedicinal survey of Orchha wildlife sanctuary region of Tikamgarh District, Madhya Pradesh, India. J. Bot. Res. 2013, 4, 31–34. [Google Scholar]
- Joshi, R.K.; Setzer, W.N.; Da Silva, J.K. Phytoconstituents, traditional medicinal uses and bioactivities of Tulsi (Ocimum sanctum Linn.): A review. Am. J. Essent. Oil Nat. Prod. 2017, 5, 18–21. [Google Scholar]
- Iqbal Chowdhury, I.; Rahman, M.A.; Hashem, M.A.; Bhuiyan, M.M.H.; Hajjar, D.; Alelwani, W.; Makki, A.A.; Haque, M.A.; Tangpong, J.; Bakhtiar, M.T.B. Supplements of an aqueous combination of Justicia adhatoda and Ocimum tenuiflorum boost antioxidative effects and impede hyperlipidemia. Anim. Models Exp. Med. 2020, 3, 140–151. [Google Scholar] [CrossRef]
- Zaman, K.A.; Khalid, A.A. Free radical scavenging activity of some Bangladeshi medicinal plants. Pharmacologyonline 2015, 3, 29–32. [Google Scholar]
- Venkatachalapathi, A.; Sangeeth, T.; Ali, M.A.; Tamilselvi, S.S.; Paulsamy, S.; Al-Hemaidc, F.M. Ethnomedicinal assessment of Irula tribes of Walayar valley of Southern Western Ghats, India. Saudi J. Biol. Sci. 2018, 25, 760–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.; Prasad, M.; Sah, N.K. Anticancer biology of Azadirachta indica L. (neem): A mini review. Cancer Biol. Ther. 2011, 12, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, I.V.S.; Neelima, P. Neem (Azadirachta indica): A Review on Medicinal Kalpavriksha. Int. J. Econ. Plants 2022, 9, 59–63. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Doley, R.; Saikia, D. Isolation of a snake venom phospholipase A2 (PLA2) inhibitor (AIPLAI) from leaves of Azadirachta indica (Neem): Mechanism of PLA2 inhibition by AIPLAI in vitro condition. Toxicon 2008, 51, 1548–1553. [Google Scholar] [CrossRef]
- Sani, I.; Umar, R.A.; Hassan, S.W.; Faruq, U.Z.; Bello, F.; Aminu, H.; Sulaiman, A. Hepatoprotective effect of Azadirachta indica leaf fractionated extracts against snake venom toxicity on albino rats. Saudi J. Biomed. Res. 2020, 5, 112–117. [Google Scholar] [CrossRef]
- Sani, I.; Umar, R.A.; Hassan, S.W.; Faruq, U.Z.; Bello, F.; Abdulhamid, A. Inhibition of snake venom enzymes and antivenom adjuvant effects of Azadirachta indica A. Juss. (Meliaceae) leaf extracts. European J. Med. Plants 2020, 31, 114–128. [Google Scholar] [CrossRef]
- Arafa, N.M.S.; Mubarak, S.A. Antivenom Activity Exploration of Azadirachta indica. Cienc. Tec. Vitivinic. 2017, 32, 110–125. [Google Scholar]
- Shrirangasami, S.R.; Murugaragavan, R.; Rakesh, S.S.; Ramesh, P.T. Chemistry behind in neem (Azadirachta indica) as medicinal value to living forms—A review. J. Pharmacogn. Phytochem. 2020, 9, 467–469. [Google Scholar] [CrossRef]
- Dagar, P.; Mishra, A. Molecular docking analysis of modified gedunin from neem with snake venom enzymes. Bioinformation 2021, 17, 776–783. [Google Scholar] [CrossRef]
- Vasudev, S.; More, V.S.; Ananthraju, K.S.; More, S.S. Potential of herbal cocktail of medicinal plant extracts against ‘big four’snake venoms from India. J. Ayurveda Integr. Med. 2021, 12, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bisht, G.S. Some novel folk treatments among the tribes of Uttar Pradesh. Anc. Sci. Life 1999, 18, 250–253. [Google Scholar] [PubMed]
- Tahir, A.; Khan, N.A.; Mansha, M.Z.; Ikram, K.; Aslam, H.M.U.; Aatif, H.M.; Hanif, C.M.S.; Ashfaq, M. Yield analysis of oyster mashroom (Pleurotus ostreatus) on Ficus religiosa leaves in combination with agricultural waste materials. Pure Appl. Biol. 2021, 10, 12–18. [Google Scholar] [CrossRef]
- Tarun, S.; Ramamurthy, A.; Parul, A.; Malvika, S. Medicinal Plants Used In Various Indian Traditional Customs. Int. J. Ayurvedic Herb. Med. 2016, 6, 2326–2332. [Google Scholar]
- Lim, T.K. Musa acuminata (AAA Group) ‘Dwarf Cavendish’. In Edible Medicinal and Non-Medicinal Plants; Springer: Amsterdam, The Netherlands, 2012; Volume 3, pp. 502–527. [Google Scholar] [CrossRef]
- Borges, M.H.; Alves, D.L.F.; Raslan, D.S.; Piló-Veloso, D.; Rodrigues, V.M.; Homsi-Brandeburgo, M.I.; De Lima, M.E. Neutralizing properties of Musa paradisiaca L. (Musaceae) juice on phospholipase A2, myotoxic, hemorrhagic and lethal activities of crotalidae venoms. J. Ethnopharmacol. 2005, 98, 21–29. [Google Scholar] [CrossRef]
- Zeeshan, U.; Barkat, M.Q.; Mahmood, H.K. Phytochemical and antioxidant screening of Cassia angustifolia, Curcuma zedoaria, Embelia ribes, Piper nigrum, Rosa damascena, Terminalia belerica, Terminalia chebula, Zingiber officinale and their effect on stomach and liver. Matrix Sci. Pharm. 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Pandey, A.K.; Shukla, P.K. Role of Medicinal Plants in Health Care and Rural Economy in the Tribals of Satpura Plateau Region of Central India. Indian For. 2008, 134, 1438–1446. [Google Scholar]
- Shankar, R.; Lavekar, G.S.; Deb, S.; Sharma, B.K.; Rawat, M.S. Distribution, conservation and folk uses of Vaibidang (Embelia ribes Burm. f.). Int. J. Biodivers. Conserv. 2012, 4, 525–529. [Google Scholar] [CrossRef]
- Arya, V.; Kumari, S.; Sharma, S. Plants Used in Reptile Bites with Emphasis on Snake Bite. Rasamruta 2015, 7, 1435778196. [Google Scholar]
- Gupta, J.; Ali, M. Chemical constituents of Boerhavia diffusa Linn. roots. Indian J. Chem. 1998, 37B, 912–917. [Google Scholar]
- Mishra, S.; Aeri, V.; Gaur, P.K.; Jachak, S.M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. Biomed. Res. Int. 2014, 2014, 808302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, G.; Rajkumar, V.; Mathew, L.; Kumar, A. The antioxidant and DNA protection potential of Indian tribal medicinal plants. Turk. J. Biol. 2011, 35, 233–242. [Google Scholar] [CrossRef]
- Sandey, H.; Sharma, L. Phytosociological study of ethno medicinal leafy vegetable flora of district Kondagaon Chhattisgarh. J. Emerg. Technol. Innov. Res. 2019, 6, 422–435. [Google Scholar]
- Giresha, A.S.; Pramod, S.N.; Sathisha, A.; Dharmappa, K. Neutralization of Inflammation by Inhibiting In vitro and In vivo Secretory Phospholipase A2 by Ethanol Extract of Boerhaavia diffusa L. Pharmacogn. Res. 2017, 9, 174–181. [Google Scholar] [CrossRef]
- Hegde, D.M. Sesame. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, p. 479. [Google Scholar] [CrossRef]
- Nagori, K.; Singh, M.K.; Alexander, A.; Kumar, T.; Dewangan, D.; Badwaik, H.; Tripathi, D. Piper betle L.: A review on its ethnobotany, phytochemistry, pharmacological profile and profiling by new hyphenated technique DART-MS (Direct Analysis in Real Time Mass Spectrometry). J. Pharm. Res. 2011, 4, 2991–2997. [Google Scholar]
- Upasani, S.V.; Beldar, V.G.; Tatiya, A.U.; Upasani, M.S.; Surana, S.J.; Patil, D.S. Ethnomedicinal plants used for snakebite in India: A brief overview. Integr. Med. Res. 2017, 6, 114–130. [Google Scholar] [CrossRef]
- Dasgupta, A.; Datta, P. Medicinal species of Piper, pharmacognostic delimitation. Q. J. Crude Drug Res. 1980, 18, 17–25. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R.; Agarwal, S.K.; Dhar, J.D. Antifertility activity of Piper longum Linn. in female rats. Nat. Prod. Res. 2006, 20, 235–239. [Google Scholar] [CrossRef]
- Biswas, K.R.; Khan, T.; Monalisa, M.N.; Swarna, A.; Ishika, T.; Rahman, M.; Rahmatullah, M. Medicinal plants used by folk medicinal practitioners of four adjoining villages of Narail and Jessore districts, Bangladesh. Am. Eurasian J. Sustain. Agric. 2011, 5, 23–33. [Google Scholar]
- Raman, R.; Raman, A.; Manohar, P.R. The arsenic and mercury-containing Tanjore pills used in treating snake bites in the 18th century Madras Presidency. Curr. Sci. 2014, 106, 1759–1763. [Google Scholar]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Chaudhari, G.S. A review on Plumbago zeylanica Linn.—A divine medicinal plant. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 119–127. [Google Scholar]
- Singh, U.P.; Yadav, P.; Goutam, M.P. Antimicrobial Efficacy of Traditionally Used Plant Chitrak Plumbago zeylanica. Asian Man 2018, 12, 161–172. [Google Scholar] [CrossRef]
- Shivanna, M.B.; Mangala, K.R.; Parinitha, M. Ethno-medicinal knowledge of Lambani community in Chikmagalur district of Karnataka, India. J. Med. Aromat. Plant Sci. 2008, 30, 105–108. [Google Scholar]
- Bhavya, J.; Vineetha, M.S.; More, V.S.; Zameer, F.; Muddapur, U.; More, S.S.; Govindappa, M. Ethnomedicinal plants and isolated compounds against Snake venom activity: A review. Indian J. Nat. Prod. Resour. 2022, 12, 491–505. [Google Scholar]
- Mandal, S.; Rath, J. Phytochemical and antioxidant activities of ethno-medicinal plants used by Fisher folks of Chilika lagoon for Indigenous Phytotherapy. J. Pharmacogn. Phytochem. 2015, 3, 55–65. [Google Scholar]
- Eshwara, J.H.K.; Kulathunga, R.D.H.; Gunarathna, E.D.T.P. Standardization of Nagaraja Guliya; A Sri Lankan Traditional Formula Used in Poisons of Animal Origin (Jangama Visha). Asian J. Adv. Res. Rep. 2020, 13, 49–59. [Google Scholar] [CrossRef]
- Jiji Mol, V.C.; Shanmugapriya, P. Scientific validation on Siddha purification process of Nabhi. Int. J. Pharm. Sci. Res. 2017, 8, 1790–1795. [Google Scholar] [CrossRef]
- Dhaliya, R.; Patil, S.; Netravathi, A. Evaluation of Effect of Vatsanabha (Aconitum Ferox Wall.) As a Prativisha against Cobra Venom (Naja naja) Toxicity: An Experimental Study. Indian J. Anc. Med. Yoga 2018, 11, 57–65. [Google Scholar] [CrossRef]
- Tsering, J.; Tag, H.; Gogoi, B.J.; Veer, V. Traditional anti-poison plants used by the Monpa tribe of Arunachal Pradesh. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization; Veer, V., Gopalakrishnan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–203. [Google Scholar] [CrossRef]
- Bhar, K.; Mondal, S.; Suresh, P. An eye-catching review of Aegle marmelos L. (Golden Apple). Pharmacogn. J. 2019, 11, 207–224. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R. Ethnobotanical studies of Bael (Aegle marmelos): A sacred plant of Hindus. Int. J. Herb. Med. 2016, 4, 114–115. [Google Scholar]
- Bhatti, R.; Singh, J.; Saxena, A.K.; Suri, N.; Ishar, M.P.S. Pharmacognostic standardisation and antiproliferative activity of Aegle marmelos (L.) Correa leaves in various human cancer cell lines. Indian J. Pharm. Sci. 2013, 75, 628–634. [Google Scholar] [PubMed]
- Singh, E.A.; Kamble, S.Y.; Bipinraj, N.K.; Jagtap, S.D. Medicinal plants used by the Thakar tribes of Raigad district, Maharashtra for the treatment of snake-bite and scorpion-bite. Int. J. Phytother. Res. 2012, 2, 26–35. [Google Scholar]
- Mali, S.S.; Dhumal, R.L.; Havaldar, V.D.; Shinde, S.S.; Jadhav, N.Y.; Gaikwad, B.S. A systematic review on Aegle marmelos (Bael). Res. J. Pharmacogn. Phytochem. 2020, 12, 31–36. [Google Scholar] [CrossRef]
- Malviya, R.; Kumar, A.; Singh, A.; Kulkarni, G.T. Pharmacological screening, Ayurvedic values and commercial utility of Aegle marmelos. Int. J. Drug Dev. Res. 2012, 4, 28–37. [Google Scholar]
- Yogesh, G.; Jha, A.K. Ethnobotanical Studies of Sacred Aegle marmelos Plant. Int. J. Adv. Res. Ideas Innov. Technol. 2017, 3, 498–501. [Google Scholar]
- Nisha, N.C.; Sreekumar, S.; Evans, D.A.; Biju, C.K. In vitro and in silico validation of anti-cobra venom activity and identification of lead molecules in Aegle marmelos (L.) Correa. Curr. Sci. 2018, 114, 1214–1221. [Google Scholar] [CrossRef]
- Taleballa, L.H. Stimulation of Lime’s (Citrus aurantifolia) Seedlings Growth by Macro and Micro-nutrients, Hormones and Botanical Treatments. Master’s Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2019. [Google Scholar]
- Sharma, A. A Study of Ethnobotany with Reference to Traditional Knowledge of India. Int. J. Multidiscip. Res. Sci. Eng. Technol. 2018, 1, 51–57. [Google Scholar]
- Mali, P.R. Ethnobotanical studies of Peth and Trimbakeshwar district Nashik, Maharashtra, India. Trends Life Sci. 2012, 1, 35–37. [Google Scholar]
- Ediriweera, E.R.H.S.S.; Premakeerthi, W.M.S.A.; Perera, A.M.H.Y. A literary review on Sapindus trifoliatus (Gaspenela) and its medicinal values. Int. J. Ayurveda Pharm. Res. 2021, 9, 51–55. [Google Scholar] [CrossRef]
- Gandhi, A.; Adiga, A.; Hegde, P.L.; Pradeep. Comprehensive review on Arishtaka (Sapindus trifoliatus L. and S. mukorossi Gaertn). J. Ayurveda Integr. Med. Sci. 2022, 7, 158–164. [Google Scholar]
- Ntume, R.; Anywar, G.U. Ethnopharmacological survey of medicinal plants used in the treatment of snakebites in Central Uganda. Curr. Life Sci. 2015, 1, 6–14. [Google Scholar]
- Kishore, K. Monograph of tobacco (Nicotiana tabacum). Indian J. Drugs 2014, 2, 5–23. [Google Scholar]
- Bharathi, Y.; Jaffarbasha, S.; Manjunath, J. Variability, correlation and path analysis for seed yield and its component traits in Bidi Tobacco (Nicotiana tabacum L.). J. Res. PJTSAU 2018, 46, 99–102. [Google Scholar]
- Bhattacharjee, P.; Bhattacharyya, D. Medicinal plants as snake venom antidotes. J. Exp. Appl. Anim. Res. 2013, 1, 156–181. [Google Scholar] [CrossRef]
- Ameya, G.; Manilal, A.; Merdekios, B. In vitro antibacterial activity and phytochemical analysis of Nicotiana tabacum L. extracted in different organic solvents. Open Microbiol. J. 2017, 11, 352–359. [Google Scholar] [CrossRef]
- Binorkar, S.V.; Jani, D.K. Traditional Medicinal Usage of Tobacco—A Review. Spatula DD 2012, 2, 127–134. [Google Scholar] [CrossRef]
- Velayudhan, K.C.; Dikshit, N.; Nizar, M.A. Ethnobotany of turmeric (Curcuma longa L.). Indian J. Tradit. Knowl. 2012, 11, 607–614. [Google Scholar]
- Singh, S.; Sankar, B.; Rajesh, S.; Sahoo, K.; Subudhi, E.; Nayak, S. Chemical composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. J. Essent. Oil Res. 2011, 23, 11–18. [Google Scholar] [CrossRef]
- Chethankumar, M.; Srinivas, L. New biological activity against phospholipase A2 by Turmerin, a protein from Curcuma longa L. J. Biol. Chem. 2008, 389, 299–303. [Google Scholar] [CrossRef]
- Ferreira, L.A.F.; Henriques, O.B.; Andreoni, A.A.S.; Vital, G.R.F.; Campos, M.M.C.; Habermehl, G.G.; de Moraes, V.L.G. Antivenom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae). Toxicon 1992, 30, 1211–1218. [Google Scholar] [CrossRef]
- Dileep, K.V.; Tintu, I.; Sadasivan, C. Molecular docking studies of curcumin analogs with phospholipase A2. Interdiscip. Sci. Comput. Life Sci. 2011, 3, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Majumdar, R.S.; Alam, P. Systematics evaluations of morphological traits, chemical composition, and antimicrobial properties of selected varieties of Elettaria cardamomum (L.) Maton. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dattatray, S.; Kamini, B.; Mohanlal, J. The Queen of Spices and Ayurveda: A brief review. Int. J. Res. Ayurveda Pharm. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Upasani, M.S.; Upasani, S.V.; Beldar, V.G.; Beldar, C.G.; Gujarathi, P.P. Infrequent use of medicinal plants from India in snakebite treatment. Integr. Med. Res. 2018, 7, 9–26. [Google Scholar] [CrossRef]
- Raj, L.F.A.; Jayalakshmy, E. Biosynthesis and characterization of zinc oxide nanoparticles using root extract of Zingiber officinale. Orient. J. Chem. 2015, 31, 51–56. [Google Scholar] [CrossRef]
- Grys, A.; Łowicki, Z.; Parus, A. Medicinal properties of ginger (Zingiber officinale Roscoe). Postępy Fitoter. 2010, 2010, 42–45. [Google Scholar]
- Selvanayagam, Z.E.; Gnanavendhan, S.G.; Balakrishna, K.; Rao, R.B. Antisnake venom botanicals from ethnomedicine. J. Herbs Spices Med. Plants 1995, 2, 45–100. [Google Scholar] [CrossRef]
- Das, A.; Dan, V.M.; Varughese, G.; Varma, A. Roots of Medicinal Importance. In Root Engineering: Basic and Applied Concepts; Morte, A., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 443–467. [Google Scholar] [CrossRef]
- Trim, S.A.; Trim, C.M.; Williams, H.F.; Vaiyapuri, S. The failures of ethnobotany and phytomedicine in delivering novel treatments for snakebite envenomation. Toxins 2020, 12, 774. [Google Scholar] [CrossRef]
- Komives, C.F.; Sanchez, E.E.; Rathore, A.S.; White, B.; Balderrama, M.; Suntravat, M.; Cifelli, A.; Joshi, V. Opossum peptide that can neutralize rattlesnake venom is expressed in Escherichia coli. Biotechnol. Prog. 2017, 33, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Tripathi, J.S.; Rai, N.P. An appraisal of the bioavailability enhancers in Ayurveda in the light of recent pharmacological advances. Ayu 2016, 37, 3. [Google Scholar] [CrossRef] [PubMed]
- Cupo, P.; Azevedo-Marques, M.M.; de Menezes, J.B.; Hering, S.E. Immediate hypersensitivity reactions after intravenous use of antivenin sera: Prognostic value of intradermal sensitivity tests. Rev. Inst. Med. Trop. Sao Paulo 1991, 33, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Progress in the Characterization of Venoms and Standardization of Antivenoms; World Health Organization: Geneva, Switzerland, 1981. [Google Scholar]
- Laxme, R.R.S.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLOS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef]
- Phillips, C.M. Sea snake envenomation. Dermatol. Ther. 2002, 15, 58–61. [Google Scholar] [CrossRef]
- Bhise, S.B.; Bhide, M.B. Presynaptic action of Hydrophis cyanocinctus venom. Bull. Haffkine Inst. 1978, 6, 92–95. [Google Scholar]
- Ehlert, U.; Gaab, J.; Heinrichs, M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus-pituitary-adrenal axis. Biol. Psychol. 2001, 57, 141–152. [Google Scholar] [CrossRef]



| Formulation/Dose/Direction | Plant Common Name | Botanical Name (Family) |
|---|---|---|
| To treat cobra, Russell’s viper or saw-scaled viper envenomation, juice of Indian snakeroot should be orally administered [20] (V. 3, p. 417). | Indian snakeroot | Rauvolfia serpentina (L.) Benth. ex Kurz (Apocynaceae) |
| Four parts of castor leaf juice diluted with one part of water should be taken orally, and a paste of the leaves should be applied to the bite area. The individual will vomit venom [20] (V. 1, p. 54). | Castor | Ricinus communis L. (Euphorbiaceae) |
| Prickly pear root levigated in cow milk should be administered twice daily. Eating spicy foods should be avoided [20] (V. 4, p. 258). | Prickly pear | Opuntia elatior Mill. (Cactaceae) |
| Sacred tree root levigated with water should be orally administered and a thick paste should be applied topically to the bite area [20] (V. 4, p. 273). | Sacred tree | Butea monosperma (Lam.) Taub. (Fabaceae) |
| To treat krait venom: 5–10 mL of Portia bark juice should be taken orally [20] (V. 4, p. 279). | Portia tree | Thespesia populnea (L.) Sol. ex Corrêa (Malvaceae) |
| To treat cobra and saw-scaled viper venom: The juice of crowded-flower jasmine leaves should be administered orally as per tolerance [20] (V. 5, p. 381). | Crowded-flower jasmine | Jasminum coarctatum Roxb. (Oleaceae) |
| To treat snake or Russell’s viper venom: Levigated paste of white Nerium root should be applied topically on the bite area, or juice of leaves should be administered orally. In case of drowsiness due to this medication, clarified butter should be administered [20] (V. 2, p. 69). | Oleander or Nerium | Nerium oleander L. (Apocynaceae) |
| To treat krait envenomation: Levigated paste of spiny gourd tubers in honey should be instilled into the eyes, or juice should be administered orally [20] (V. 2, p. 74). | Spiny gourd | Momordica dioica Roxb. ex Willd. (Cucurbitaceae) |
| Levigated paste of spiny gourd tubers in water should be administered orally and applied topically to the stung area [20] (V. 2, p. 74). | Spiny gourd | Momordica dioica Roxb. ex Willd. (Cucurbitaceae) |
| Apple of Sodom leaves should be crushed with its sticky sap and formed into pills. These pills should be administered orally at regular intervals, or a levigated root paste should be administered orally [20] (V. 5, p. 360). | Apple of Sodom | Calotropis procera Aiton (Apocynaceae) |
| Levigated paste of sacred tree root should be administered orally or applied topically [20] (V. 4, p. 273). | Sacred tree | Butea monosperma (Lam.) Taub. (Fabaceae) |
| Levigated paste of creeping launaea root should be administered orally [20] (V. 4, p. 278). | Creeping launaea | Launaea procumbens L. (Asteraceae) |
| Colocynth root should be consumed in Paan/Vida (a preparation of betel leaf and areca nut made with slacked lime) [20] (V. 1, p. 38). | Colocynth | Citrullus colocynthis L. (Cucurbitaceae) |
| Levigated paste of conkerberry root in water should be administered orally [20] (V. 2, p. 75). | Conkerberry | Carissa congesta Wight. (Apocynaceae) |
| Filtered aqueous soapberry solution should be instilled in the eyes. In the case of severe envenomation, soapberry water should be administered orally so that the venom is vomited out. To avoid postinstillation irritation of the eyes, white butter or clarified butter should be applied [20] (V. 5, p. 357). | Indian soapberry | Sapindus mukorossi Gaertn. (Sapindaceae) |
| Snakebite test: A person not recognising the taste of neem leaves, salt or chilli peppers when given orally indicates snake envenomation. To treat envenomation, neem leaves should be chewed, or leaf or bark juice should be administered orally [20] (V. 2, p. 63). | Neem | Azadirachta indica A.Juss. (Meliaceae) |
| In the case of skin lumps caused by saw-scaled viper venom: Warmed bitter cumin leaves should be applied topically, or its juice should be rubbed on the affected area [20] (V. 2, p. 61). | Bitter cumin | Centratherum anthelminticum (L.) Kuntze. (Asteraceae) |
| For cobra venom: Cluster fig bark paste diluted with the mixture of its juice and milk should be administered orally [9] (p. 89). | Cluster fig | Ficus racemosa L. (Moraceae) |
| In the event of a snakebite: Jaggery and sesame seeds should be crushed in cow’s milk and consumed orally [9] (p. 16). | Sesame seeds | Sesamum indicum L. (Pedaliaceae) |
| Crushed paste of prickly pear leaves should be applied topically [9] (p. 87). | Prickly pear | Opuntia elatior Mill. (Cactaceae) |
| Punarnava root levigated with water should be administered [9] (p. 87). | Punarnava | Boerhavia diffusa L. Nom. Cons. (Nyctaginaceae) |
| Powdered Punarnava roots should be administered orally with water [9] (p. 87). | Punarnava | Boerhavia diffusa L. Nom. Cons. (Nyctaginaceae) |
| Crushed coffeeweed root paste should be instilled in the eyes [9] (p. 99). | Coffeeweed | Cassia occidentalis (L.) Rose. (Caesalpiniaceae) |
| The root of the apple of Sodom levigated with water should be instilled into the nose and eyes [9] (p. 99). | Apple of Sodom | Calotropis procera Aiton (Apocynaceae) |
| Rosary peas levigated with water should be administered orally [9] (p. 99). | Rosary pea | Abrus precatorius L. (Fabaceae) |
| An aqueous solution of Indian soapberry should be administered orally until the patient vomits a couple of times. An aqueous paste made from Indian soapberry should be instilled into the eyes. Levigated paste of Indian soapberries should be applied topically to the stung area [9] (p. 15). | Indian soapberry | Sapindus mukorossi Gaertn. (Sapindaceae) |
| Powdered potato yam stem bark should be administered with water [9] (p. 19). | Potato yam | Dioscorea bulbifera L. (Dioscoreaceae) |
| A reference from Ceylon (Sri Lanka): The key lime juice was applied to the stung area, and leeches were placed around it. On the third day, a laxative was administered, and the person was cured [9] (p. 114). | Key lime | Citrus aurantifolia (Christm.) Swingle (Rutaceae) |
| One or one and a half bristly luffa fruits should be crushed in water, decanted and administered orally. Clarified butter should be administered after vomiting occurs [9] (p. 124). | Bristly luffa | Luffa echinata Roxb. (Cucurbitaceae) |
| To treat Russel’s viper bite: Apply castor oil topically on the bite [9] (p. 142). | Castor | Ricinus communis L. (Euphorbiaceae) |
| To treat krait envenomation: Oral administration of fire-flame bush juice is recommended [9] (p. 256). | Fire-flame bush | Woodfordia fruticosa (L.) Kurz (Lythraceae) |
| The juice prepared from a minimum of 12–18 g of colocynth or its water extract should be taken orally. Venom will be expelled out in the form of vomit or faeces [9] (p. 260). | Colocynth | Citrullus colocynthis L. (Cucurbitaceae) |
| The root of Indian night shade levigated with water should be administered orally and applied topically on the stung area [9] (p. 274). | Indian night shade | Solanum indicum L. (Solanaceae) |
| Bristly luffa fruit extract in 40 g of cold water should be taken orally twice a day [9] (p. 297). | Bristly luffa | Luffa echinata Roxb. (Cucurbitaceae) |
| Levigated paste of devil’s goad roots should be administered orally [9] (p. 297). | Devil’s goad | Croton roxburghii Balakr. (Euphorbiaceae) |
| Steamed grated unripe papaya should be applied topically for 2–4 days around the bite area [9] (p. 297). | Papaya | Carica papaya L. (Caricaceae) |
| Formulation/Dose/Direction | Plant Common Name | Botanical Name (Family) |
|---|---|---|
| Levigated paste of Ceylon Leadwort roots, chilla roots and Kale Vel bulb should be administered thrice orally at intervals. The snakebite victim should be made to sit on a platform made up of cow dung while a stream of cold water should be poured on their body. The effects of the venom would wear off in about 6 h. In case of adverse events, clarified butter can be orally administered [20] (V. 3, p. 164). | Ceylon leadwort | Plumbago zeylanica L. (Plumbaginaceae) |
| Chilla | Casearia graveolens Dalz. (Salicaceae) | |
| Kale Vel | Unidentified | |
| Freshwater mangrove fruits levigated with garlic juice should be instilled into the eyes [20] (V. 6, p. 403). | Freshwater mangrove | Barringtonia acutangula (L.) Gaertn. (Lecythidaceae) |
| Garlic | Allium sativum L. (Amaryllidaceae) | |
| A pill should be made from finely powdered purging croton seed kernels that have been coated with key lime fruit juice 12 times. This pill levigated with human saliva should be instilled into the eyes [20] (V. 3, p. 175). | Purging croton | Croton tiglium L. (Euphorbiaceae) |
| Key lime | Citrus aurantifolia (Christm.) Swingle (Rutaceae) | |
| A diluted paste of Indian birthwort made with soapberries or white abrus should be taken orally at regular intervals [20] (V. 6, p. 418). | Indian birthwort | Aristolochia indica L. (Aristolochiaceae) |
| Indian soapberry | Sapindus mukorossi Gaertn. (Sapindaceae) | |
| White abrus | Abrus precatorius L. (Fabaceae) | |
| To treat Russell’s viper envenomation: A thick paste of devil’s goad roots, soapberries, and bitter cumin made with veld grape juice should be diluted with cow urine and applied topically at the bite site [20] (V. 3, p. 155). | Veld grape | Cissus quadrangularis L. (Vitaceae) |
| Devil’s goad | Croton roxburghii Balakr. (Euphorbiaceae) | |
| Indian soapberry | Sapindus mukorossi Gaertn. (Sapindaceae) | |
| Bitter cumin | Centratherum anthelminticum (L.) Kuntze. (Asteraceae) | |
| To treat Russell’s viper and krait venom: Paste formed by levigating devil’s goad roots and dry ginger should be administered orally. It will act as purgative and emetic. In case of skin lumps due to envenomation, the levigated paste of devil’s goad roots should be applied topically [20] (V. 3, p. 152). | Devil’s goad | Croton roxburghii Balakr. (Euphorbiaceae) |
| Dry ginger | Zingiber officinale Roscoe (Zingiberaceae) | |
| A finely powdered mixture of dried white Nerium flowers, tobacco and cardamom should be insufflated [20] (V. 2, p. 69). | Nerium | Nerium oleander L. (Apocynaceae) |
| Tobacco | Nicotiana tabacum L. (Solanaceae) | |
| Cardamom | Elettaria cardamomum L. Maton (Zingiberaceae) | |
| To treat Russell’s viper venom: Levigated paste of devil’s goad roots in the juice of three parts hog plum bark, two parts grey downy balsam bark and one part frangipani bark should be administered orally as tolerated [20] (V. 1, p. 16). | Devil’s goad | Croton roxburghii Balakr. (Euphorbiaceae) |
| Hog plum | Spondias pinnata (L.f.) Kurz. (Anacardiaceae) | |
| Grey downy balsam | Garuga pinnata Roxb. (Burseraceae) | |
| Frangipani | Plumeria acutifolia Poir. (Apocynaceae) | |
| Jaggery and sesame seeds should be crushed in the sticky sap of apple of Sodom and consumed orally [9] (p. 95). | Sesame seeds | Sesamum indicum L. (Pedaliaceae) |
| Apple of Sodom | Calotropis procera Aiton (Apocynaceae) | |
| Powdered East Indian walnut seeds mixed with prickly pear sticky sap should be applied to the stung area [9] (p. 156). | East Indian walnut | Albizia saman (Jacq.) F.Muell. (Fabaceae) |
| Prickly pear | Opuntia elatior Mill. (Cactaceae) | |
| Equal portions of salted dried roselle, powdered limestone and powdered turmeric should be levigated in cow urine and applied topically on the bite area for 3–7 days [9] (p. 156). | Roselle | Hibiscus sabdariffa L. (Malvaceae) |
| Turmeric | Curcuma longa L. (Zingiberaceae) | |
| A person fainted as a result of snake envenomation. An ascetic rubbed holy basil juice around the victim’s navel, chest, and forehead and kept a cloth ball dipped in the holy basil juice until the victim woke up. The banana stem water was then administered orally [9] (p. 196). | Holy basil/Tulasi | Ocimum tenuiflorum L. (Lamiaceae) |
| Banana | Musa acuminata Colla (Musaceae) | |
| An amount of 30 g of Bengal quince bark or leaves juice, 30 g of Frangipani bark juice, and 3 pills of Sanjeevini should be administered together [9] (p. 297). | Bengal quince | Aegle marmelos (L.) Corr. (Rutaceae) |
| Frangipani | Plumeria acutifolia Poir. (Apocynaceae) | |
| Sanjeevini | Selaginella bryopteris (L.) Baker (Selaginellaceae) |
| Common Name | Botanical Name (Family) |
|---|---|
| Aconite | Aconitum heterophyllum Wall. (Ranunculaceae) |
| Asafoetida | Ferula assa-foetida L. (Apiaceae) |
| Babreng | Embelia ribes Burm.f. (Myrsinaceae) |
| Betle leaf | Piper betle L. (Piperaceae) |
| Black pepper | Piper nigrum L. (Piperaceae) |
| Castor | Ricinus communis L. (Euphorbiaceae) |
| Ceylon leadwort | Plumbago zeylanica L. (Plumbaginaceae) |
| Chebulic myrobalan | Terminalia chebula Retz. (Combretaceae) |
| False daisy/Bhringraj | Eclipta prostrata L. (Asteraceae) |
| Freshwater mangrove | Barringtonia acutangula (L.) Gaertn. (Lecythidaceae) |
| Garlic | Allium sativum L. (Amaryllidaceae) |
| Ginger | Zingiber officinale Roscoe (Zingiberaceae) |
| Henbane | Hyoscyamus niger L. (Solanaceae) |
| Holy basil/Tulasi | Ocimum tenuiflorum L. (Lamiaceae) |
| Indian aconite | Aconitum ferox Wall. ex Ser. (Ranunculaceae) |
| Indian barberry | Berberis aristata DC. (Berberidaceae) |
| Indian gooseberry | Phyllanthus emblica L. (Euphorbiaceae) |
| Liquorice | Glycyrrhiza glabra L. (Fabaceae) |
| Long pepper | Piper longum L. (Piperaceae) |
| Mount Atlas daisy | Anacyclus pyrethrum (L.) Link. (Asteraceae) |
| Nutmeg, Mace | Myristica fragrans Houtt. (Myristicaceae) |
| Opium poppy | Papaver somniferum L. (Papaveraceae) |
| Purging croton | Croton tiglium L. (Euphorbiaceae) |
| Pushkarmool | Inula racemosa Hook.f. (Asteraceae) |
| Sacred fig | Ficus religiosa L. (Moraceae) |
| Family | Botanical Name | Ethnobotanical Evidence (Plant Part) $ | Pharmacological Evidence |
|---|---|---|---|
| Amaryllidaceae | |||
| Allium sativum L. | Bulbs [18,56,57,58,59] | Ethanolic, methanolic and aqueous extracts of garlic (Allium sativum) when tested against Naja naja karachiensis venom by in vitro (hen’s egg yolk mixture) and in vivo (male rabbits) methods; Phospholipase A2 and snake venom proteins were neutralized, and snake venom proteins failed to bind to their potential targets due to hindrance by secondary metabolites of garlic [60,61,62]. Orally administered dose of garlic juice to rats for 10 days can be used as a prophylactic tool against cobra venom without significant side effects on gastric and hepatic tissues [63]. Garlic bulbs neutralized coagulant, fibrinolytic and phospholipase activity in vitro from Naja naja venom [64]. | |
| Anacardiaceae | |||
| Spondias pinnata (L.f.) Kurz. Spondias mangifera Willd. | Leaves, Fruits [65] Plant * [12] | None | |
| Apiaceae | |||
| Ferula assa-foetida L. | Exudates [66] | None | |
| Apocynaceae | |||
| Calotropis procera Aiton Calotropis gigantia L. Dryand | Whole plant [67] Roots [18] Root bark [68] Leaves [69,70,71] Flowers [72] Dried latex [73] | Organic solvent extracts of Calotropis inhibited various enzymes from Naja naja venom when tested in vitro [74]. Fractionated methanolic extract of Calotropis procera neutralized the activity of 5′-nucleotidase from Echis carinatus venom by 85% when compared with snake venom antiserum [75]. Calotropis inhibited proteases and phospholipase A2 found in snake venoms when tested in vitro [76]. The methanolic root extract of Calotropis has potent inhibitors of phospholipase A2 from Daboia russelii venom [77]. Methanolic extract of Calotropis procera exhibited some degree of protection against Bitis arietans, Echis ocellatus and Naja nigricollis snake venoms in albino rats [78]. | |
| Carissa congesta Wight. Carissa carandas L. | Leaves [79,80] Plant * [81] | None | |
| Nerium oleander L. | Whole plant [69] Roots [82,83] Leaves [84,85] Seeds [86] | None | |
| Plumeria acutifolia Poir. Plumeria lutea Ruiz. Plumeria alba L. Plumeria rubra L. | Fruits [18] | None | |
| Rauvolfia serpentina (L.) Benth. ex Kurz | Whole plant [69] Roots [18,87,88,89,90] Roots, Leaves [91] Plant * [92,93] | The alkaloid fraction of Rauvolfia serpentina leaves neutralized Crotalus adamanteus snake venom by reducing venom lethality, Superoxide dismutase activity and lipid peroxidation [17]. Aqueous extract of roots of Rauvolfia serpentina neutralized enzymes such as Acetylcholinesterase, ATPase and Protease and lethality of Naja naja snake venom when conducted by in vivo and in vitro methods [94]. Rauvolfia serpentina contains molecules that can potentially inhibit various cobra venom enzymes such as Acetylcholinesterase, L-aminoacid oxidase, Phospholipase A2 and Serine protease when tested in silico via molecular docking techniques [95]. Neurotoxins from Bungarus caeruleus, Dendroaspis polylepis polylepis, Naja naja and Oxyuranus microlepidotus venoms depicted a high affinity for binding with phytochemical compounds from Rauvolfia serpentina when tested in silico via molecular docking studies [96]. | |
| Aristolochiaceae | |||
| Aristolochia indica L. | Whole plant [69,97,98,99,100] Roots [17,86,88,101,102,103,104,105] | Aqueous ethanolic extract of Aristolochia indica inhibited various enzymes from Naja naja venom when tested in vitro [74]. The alkaloid fraction of Aristolochia indica leaves neutralized Crotalus adamanteus snake venom by reducing venom lethality, Superoxide dismutase activity and lipid peroxidation [17]. Methanolic extract of Aristolochia indica neutralized lethal action of Daboia russelii, Echis carinatus, Naja kaouthia and Ophiophagus hannah venoms in male albino mice; haemorrhagic action of Daboia russelii and Echis carinatus venom in male albino mice; coagulant activity of Daboia russelii venom in vitro; defibrinogenating activity of Daboia russelii venom in male albino mice; Phospholipase A2 activity of Daboia russelii venom in vitro [104]. An aqueous root extract of Aristolochia indica elongated the duration of survival of animals after the application of Russell’s viper venom [106]. Methanolic extract of Aristolochia indica (whole plant) completely neutralized the lethality of Daboia russelli venom, and oedema, haemorrhagic, coagulant, fibrinolytic and phospholipase activities were reversed. Aristolochia indica plant extract has a potent activity to neutralize Russell’s viper venom [107]. Aristolochic acid from Aristolochia indica inhibited the in vitro activity of purified hyaluronidase from Naja naja venom as well as the overall venom hyaluronidase activity in a dose-dependent manner [108]. Mice injected with whole N. naja venom preincubated with aristolochic acid had a two-fold increase in survival time when compared to mice injected with venom alone. Aristolochic acid injection 10 min after whole venom injection resulted in a more moderate increase in survival time [108]. Aristolochic acid inhibited Phospholipase A2 from Vipera russelii venom in a noncompetitive manner [109]. A major constituent of A. indica, aristolochic acid, inhibited Russell’s viper venom L-amino acid oxidase enzyme activity by interacting with DNA [110]. | |
| Asteraceae | |||
| Centratherum anthelminticum (L.) Kuntze. | Seeds [111] | None | |
| Eclipta prostrata L. | Plant * [69,112] | The alkaloid fraction of Eclipta prostrata leaves neutralized Crotalus adamanteus snake venom by reducing venom lethality, superoxide dismutase activity and lipid peroxidation [17]. Butanolic extract of Eclipta prostrata partially inhibited the haemorrhagic activity of Calloselasma rhodostoma (Malayan pit viper) venom [113]. The lethal activity of Crotalus durissus terrficus (South American rattlesnake) venom was neutralized by ethanolic extracts of Eclipta prostrata (aerial parts) in mice when mixed in vitro before injection intraperitoneally [114]. Eclipta prostrata acts as antimyotoxic and antihaemorrhagic against crotalid venoms [115]. Eclipta prostrata ethyl acetate extract inhibited the proteolytic and haemorrhagic activity of Calloselasma rhodostoma (Malayan pit viper) venom [116]. | |
| Berberidaceae | |||
| Berberis aristata DC. | Berberine [117] Bulbs ^ [118] | None | |
| Caesalpiniaceae | |||
| Cassia occidentalis (L.) Rose. | Roots [69] | None | |
| Caricaceae | |||
| Carica papaya L. | Papain [69,119] Leaves [120] Plant * [121] | None | |
| Combretaceae | |||
| Terminalia chebula Retz. | Fruits ^ [118] Fruits ~ [122,123] | None | |
| Cucurbitaceae | |||
| Citrullus colocynthis L. | Fruits [124] Roots, Fruits [125] Roots, Fruits, Seeds [126,127] | Methanolic extract of the whole plant of Citrullus colocynthis neutralized haemorrhage induced by Naja naja karachiensis snake venom [128]. | |
| Momordica dioica Roxb. ex Willd. | Roots [18] Roots/Tubers [129] Roots, Fruits [130] Fruits [131,132] Plant * [133,134] | None | |
| Dioscoreaceae | |||
| Dioscorea bulbifera L. | Sap [135] | None | |
| Euphorbiaceae | |||
| Croton roxburghii Balakr. | Stem bark [136] Plant * [12,137,138,139] | None | |
| Croton tiglium L. | Roots, Stem Bark, Seeds [140,141] Plant * [142] | None | |
| Phyllanthus emblica L. Emblica officinalis Gaertn. | Fruits ^ [118] Plant * [143] | Fruits of Indian gooseberry when tested in vitro for Naja naja venom-neutralizing capacity by evaluating coagulant activity, fibrinolytic activity and phospholipase activity were found effective [64]. Methanolic root extract of Emblica officinalis significantly neutralized lethal activities of Naja kaouthia and Vipera russelii venoms and reversed V. russelii venom-induced coagulant, defibrinogenating, haemorrhage and inflammatory activities [144]. A phthalate compound from Emblica officinalis root extract neutralized cardiotoxic, defibrinogenating, haemorrhagic, neurotoxic, myotoxic, proinflammatory and PLA2 activities induced by viper and cobra venoms [145]. | |
| Fabaceae | |||
| Abrus precatorius L. | Roots [17,69,146,147,148,149,150,151] | None | |
| Butea monosperma (Lam.) Taub. | Roots [152,153,154] Stem bark [155] | Organic solvent extracts of Butea monosperma inhibited various enzymes from Naja naja venom when tested in vitro [74]. Ethanolic extract of Butea monosperma inhibited Vipera russelii venom hyaluronidase in a dose-dependent manner, and thus, venom-induced haemorrhage was reduced significantly [156]. | |
| Glycyrrhiza glabra L. | Roots [157] | Glycyrrhizin, from Glycyrrhiza glabra prevents Bothrops jararaca venom-induced changes in haemostasis both in vitro and in vivo [158]. Flavonoids from Glycyrrhiza glabra inhibit the activity of cytosolic and secreted phospholipase A2 in a dose-dependent manner suggesting anti-inflammatory and immunomodulatory actions when tested via in silico methods [159]. | |
| Lamiaceae | |||
| Ocimum tenuiflorum L. Ocimum sanctum L. | Flowers ^ [118] Plant * [160,161,162] | Tulsi leaves were tested in vitro for Naja naja venom neutralizing capacity by evaluating coagulant activity, fibrinolytic activity and phospholipase activity and were found effective [64]. The alkaloid fraction of Ocimum sanctum leaves neutralized Crotalus adamanteus snake venom by reducing venom lethality, superoxide dismutase activity and lipid peroxidation [17]. | |
| Lecythidaceae | |||
| Barringtonia acutangula (L.) Gaertn. | Seeds ~ [163] | None | |
| Lythraceae | |||
| Woodfordia fruticosa (L.) Kurz | Whole plant [69] Leaves [18] | None | |
| Meliaceae | |||
| Azadirachta indica A.Juss. | Whole plant [164] Leaves [69,151,165] Stem bark, Gum, Leaves, Seeds [166] | Neem bark was tested in vitro for Naja naja venom neutralizing capacity by evaluating coagulant activity, fibrinolytic activity and phospholipase activity and was found effective [64]. Organic solvent extracts of Azadirachta indica inhibited various enzymes from Naja naja venom when tested in vitro [74]. The terpenoid fraction of Azadirachta indica leaves neutralized Crotalus adamanteus snake venom by reducing venom lethality, superoxide dismutase activity and lipid peroxidation [17]. An isolated compound—AIPLAI—from methanolic leaf extract of Azadirachta indica inhibited phospholipase A2 from cobra and Russell’s viper venoms in a dose-dependent manner. AIPLAI inhibited purified phospholipase A2 from Naja kaouthia venom in a noncompetitive manner [167]. Fractionated leaf extracts of Azadirachta indica presented significant hepatoprotection against the Naja nigricollis venom-induced toxicity in albino rats [168]. Isolated organic fractions of methanolic leaf extract of Azadirachta indica significantly inhibited Naja nigricollis venom enzymes in vitro. A. indica extract acts as an effective adjuvant when used with snake venom antiserum in the treatment [169]. Aqueous and methanolic extracts of Azadirachta indica leaves are protective against Naja haje arabica and Bitis arietans arietans snake venoms in mice [170]. Leaf extract of Azadirachta indica has a clotting agent that acts against Russell’s viper venom [171]. The modified gedunin from Azadirachta indica showed improved pharmacological properties for combating snakebites in the molecular docking studies against snake venom enzymes [172]. A mixture of aqueous ethanolic extracts of Azadirachta indica, Areca catechu, Butea monosperma, Citrus limon and Clerodendrum serratum inhibited different components of Naja naja, Bungarus caeruleus, Daboia russelii and Echis carinatus venoms in different in vivo and in vitro studies [173]. | |
| Moraceae | |||
| Ficus religiosa L. | Leaves [174,175,176] Flowers [69] | None | |
| Musaceae | |||
| Musa acuminata Colla Musa x paradisiaca L. | Plant * [177] | Musa paradisiaca extract when mixed with Crotalidae venoms in vitro and administered in mice, inhibited venom lethality, haemorrhagic activity, myotoxicity and phospholipase A2 [177,178]. | |
| Myrsinaceae | |||
| Embelia ribes Burm.f. | Seeds [179,180] Plant * [181,182] | None | |
| Nyctaginaceae | |||
| Boerhavia diffusa L. Nom. Cons. | Roots [183] Leaves [184,185,186] | Ethanolic extract of Boerhavia diffusa inhibited phospholipase A2 in significant amounts [187]. | |
| Papaveraceae | |||
| Papaver somniferum L. | Plant * [69] | None | |
| Pedaliaceae | |||
| Sesamum indicum L. | Seed oil [188] | None | |
| Piperaceae | |||
| Piper betle L. | Whole Plant [189] Leaves [18] | None | |
| Piper longum L. | Roots [190] Roots, Fruits [191,192] Fruits [18] Fruits ^ [118] | Ethanolic extract of Piper longum fruits inhibited Russell’s viper venom-induced defibrinogenation, haemorrhage, inflammation, and lethality and proved to be a good antisnake venom [42]. Methanolic extract of Piper longum fruit neutralized procoagulant, proteolytic activity and phospholipase A2 from Daboia russelii venom when tested in vitro [44]. | |
| Piper nigrum L. | Fruits [193,194] Fruits ^ [118] Fruits, Seeds [190,195] Seeds [30,105] | None | |
| Plumbaginaceae | |||
| Plumbago zeylanica L. | Roots [196,197,198,199] Leaves [200] | None | |
| Ranunculaceae | |||
| Aconitum ferox Wall. ex Ser. | Roots [194,201] Rhizomes [202] | The aqueous root extract of Aconitum ferox administered orally was evaluated in mice against Naja naja venom. No effect on venom mortality was reported; however, reversal of ill effects on Lymphocyte-Neutrophil Ratio and SGOT were observed [203]. | |
| Aconitum heterophyllum Wall. | Tubers [204] | None | |
| Rutaceae | |||
| Aegle marmelos (L.) Corr. | Whole plant [205,206,207] Roots [11] Roots ^ [118] Root bark [17] Stem bark [18] Leaves [208] Roots, Leaves, Stem bark [209,210,211] | Aqueous ethanolic extract of Aegle marmelos inhibited various enzymes from Naja naja venom when tested in vitro [74]. Ethanol, methanol and hexane extracts of leaves, stem bark and root bark of Aegle marmelos were tested by in vitro and in silico methods against Naja naja venom. Venom lethality inhibitory activity, antihaemolytic activity, antiacetylcholinesterase activity and antiproteolytic activity were presented by the extracts [212]. | |
| Citrus aurantifolia (Christm.) Swingle | Fruit juice [213] | None | |
| Salicaceae | |||
| Casearia graveolens Dalz. | Twigs [214] Plant * [215] | None | |
| Sapindaceae | |||
| Sapindus mukorossi Gaertn. Sapindus laurifolius Vahl. Sapindus trifoliatus L. | Leaves, Stem bark [216] Fruits [217] | Methanolic extract of Sapindus laurifolius fruit neutralized procoagulant, proteolytic activity and phospholipase A2 from Daboia russelii venom when tested in vitro [44]. | |
| Solanaceae | |||
| Nicotiana tabacum L. | Roots, Leaves [218] Leaves [219,220] Seeds [18] Plant * [120,221,222,223] | None | |
| Zingiberaceae | |||
| Curcuma longa L. | Rhizomes [11,69,224] Rhizomes ^ [118] Essential oil from rhizome [225] | Fractionated methanolic extract of Curcuma longa neutralized activity of 5′-nucleotidase from Echis carinatus venom by 83.7% when compared with snake venom antiserum [75]. Turmerin from Curcuma longa inhibited phospholipase A2 from Naja naja (cobra) snake venom and prevented cytotoxicity, oedema and myotoxicity [226]. Ar-Turmerone from Curcuma longa neutralized haemorrhagic activity from Bothrops jararaca venom and lethal effect of Crotalus durissus terrificus venom when tested in mice [227]. The binding of curcumin analogues with phospholipase A2 was tested via molecular docking studies, and dihydrocurucmin, tetrahydrocurcumin, hexahydrocurcumin and rosmarinic acid were found to have the more binding potential [228]. | |
| Elettaria cardamomum L. Maton | Seeds [229,230,231] Seeds, Pods [232] | None | |
| Zingiber officinale Roscoe | Rhizomes [233,234,235] Rhizomes ^ [118] Rhizomes ~ [30] | None | |
| Family | References | |||
|---|---|---|---|---|
| Snake Species | Action | Enzyme X | Toxin X | |
| Elapidae | ||||
| Bungarus caeruleus | [173] | [96] | ||
| Dendroaspis polylepis polylepis | [96] | |||
| Naja haje arabica | [170] | |||
| Naja kaouthia | [104,144] | [167] | ||
| Naja naja | [64,78,145,203,212,226] | [64,74,94,95,108,145,173,212] | [96,145,226] | |
| Naja naja karachiensis | [128] | [60,61,62] | ||
| Naja nigricollis | [168,169] | [169] | ||
| Ophiophagus hannah | [104] | |||
| Oxyuranus microlepidotus | [96] | |||
| Viperidae | ||||
| Bitis arietans | [78,170] | |||
| Bothrops jararaca | [158,227] | |||
| Calloselasma rhodostoma | [113,116] | |||
| Crotalid species | [115,177,178] | [177,178] | [177,178] | |
| Crotalus adamanteus | [17] | [17] | ||
| Crotalus durissus terrificus | [114,227] | |||
| Daboia russelii | [42,44,104,106,107,144,145,156,171] | [44,77,104,107,109,110,145,156,173] | [145] | |
| Echis carinatus | [104] | [75,173] | ||
| Echis ocellatus | [78] | |||
| Unspecified snake species | [159] | [76,159,172,187,228] | ||
| Family | Plant Species |
|---|---|
| Asteraceae | Anacyclus pyrethrum (L.) Link. |
| Inula racemosa Hook.f. | |
| Launaea procumbens L. | |
| Burseraceae | Garuga pinnata Roxb. |
| Cactaceae | Opuntia elatior Mill. |
| Cucurbitaceae | Luffa echinata Roxb. |
| Euphorbiaceae | Ricinus communis L. |
| Malvaceae | Hibiscus sabdariffa L. |
| Thespesia populnea (L.) Sol. ex Corrêa | |
| Mimosaceae | Albizia saman (Jacq.) F.Muell. |
| Moraceae | Ficus racemosa L. |
| Myristicaceae | Myristica fragrans Houtt. |
| Oleaceae | Jasminum coarctatum Roxb. |
| Papaveraceae | Fumaria indica Lam. |
| Selaginellaceae | Selaginella bryopteris (L.) Baker |
| Solanaceae | Hyoscyamus niger L. |
| Solanum indicum L. | |
| Vitaceae | Cissus quadrangularis L. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshpande, A.M.; Sastry, K.V.; Bhise, S.B. A Contemporary Exploration of Traditional Indian Snake Envenomation Therapies. Trop. Med. Infect. Dis. 2022, 7, 108. https://0-doi-org.brum.beds.ac.uk/10.3390/tropicalmed7060108
Deshpande AM, Sastry KV, Bhise SB. A Contemporary Exploration of Traditional Indian Snake Envenomation Therapies. Tropical Medicine and Infectious Disease. 2022; 7(6):108. https://0-doi-org.brum.beds.ac.uk/10.3390/tropicalmed7060108
Chicago/Turabian StyleDeshpande, Adwait M., K. Venkata Sastry, and Satish B. Bhise. 2022. "A Contemporary Exploration of Traditional Indian Snake Envenomation Therapies" Tropical Medicine and Infectious Disease 7, no. 6: 108. https://0-doi-org.brum.beds.ac.uk/10.3390/tropicalmed7060108