Facile Synthesis and Surface Characterization of Titania-Incorporated Mesoporous Organosilica Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
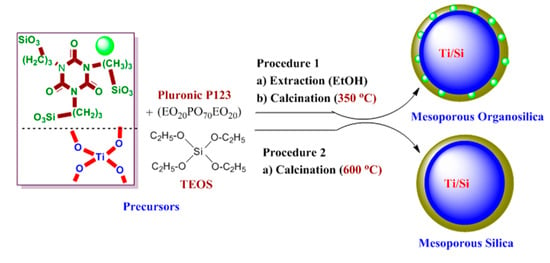
2.2. Preparation
2.3. Measurements
2.4. Solid-State NMR
2.5. Calculations
2.6. Test of Basic Properties of Materials Using CO2 Adsorption Measurement
3. Results and Discussion
3.1. Properties of Ti-MO Samples Prepared at Different Synthesis Conditions
3.2. CO2 Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Voort, P.; Vercaemst, C.; Schaubroeck, D.; Verpoort, F. Ordered mesoporous materials at the beginning of the third millennium: new strategies to create hybrid and non-siliceous variants. Phys. Chem. Chem. Phys. 2008, 10, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid inorganic-organic mesoporous silicates-nanoscopic reactors coming of age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Sayari, A.; Hamoudi, S. Periodic Mesoporous Silica-Based Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3151–3168. [Google Scholar] [CrossRef]
- Hatton, B.; Landskron, K.; Whitnall, W.; Perovic, D.; Ozin, G.A. Past, Present, and Future of Periodic Mesoporous Organosilicas. Acc. Chem. Soc. Res. 2005, 38, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechnol. 2005, 5, 347–371. [Google Scholar] [CrossRef]
- Gan, W.Y.; Chiang, K.; Brungs, M.; Amal, R.; Zhao, H. Dense TiO2 thin film: Photoelectrochemical and photocatalytic properties. Int. J. Nanotechnol. 2007, 4, 574–587. [Google Scholar] [CrossRef]
- Hoffman, F.; Cornelius, M.; Morell, J.; Froba, M. Periodic Mesoporous Organosilicas (PMOs): Past, Present, and Future. J. Nanosci. Nanotechnol. 2006, 6, 265–288. [Google Scholar] [CrossRef]
- Grudizien, R.M.; Grabicka, B.E.; Pikus, S.; Jaroniec, M. Periodic Mesoporous Organosilicas with Ethane and Large Isocyanurate Bridging Groups. Chem. Mater. 2006, 18, 1722–1725. [Google Scholar] [CrossRef]
- Gianotti, E.; Frache, A.; Coluccia, S.; Thomas, J.M.; Maschmeyer, T.; Marchese, L. The identity of titanium centres in microporous aluminophosphates compared with Ti-MCM-41 mesoporous catalyst and titanosilsesquioxane dimer molecular complex: a spectroscopy study. J. Mol. Catal. A Chem. 2003, 204, 483–489. [Google Scholar] [CrossRef]
- Hu, Y.; Higashimoto, S.; Martra, G.; Zhang, J.L.; Matsuoka, M.; Coluccia, S.; Anpo, M. Local Structures of Active Sites on Ti-MCM-41 and Their Photocatalytic Reactivity for the Decomposition of NO. Catal. Lett. 2003, 90, 161–163. [Google Scholar] [CrossRef]
- Chao, M.C.; Lin, H.P.; Mou, C.Y.; Cheng, B.W.; Cheng, C.F. Synthesis of nano-sized mesoporous silicas with metal incorporation. Catal. Today 2004, 97, 81–87. [Google Scholar] [CrossRef]
- Vinu, A.; Ariga, K.; Saravanamurugan, S.; Hartmann, M.; Murugesan, V. Synthesis of highly acidic and well ordered MgAl-MCM-41 and its catalytic performance on the isopropylation of m-cresol. Microporous Mesoporous Mater. 2004, 76, 91–98. [Google Scholar] [CrossRef]
- Alba, M.D.; Luan, Z.H.; Klinowski, J. Titanosilicate Mesoporous Molecular Sieve MCM-41: Synthesis and Characterization. J. Phys. Chem. 1996, 100, 2178–2182. [Google Scholar] [CrossRef]
- Morey, M.S.; O’Brien, S.; Schwarz, S.; Stucky, G.D. Hydrothermal and Postsynthesis Surface Modification of Cubic, MCM-48, and Ultralarge Pore SBA-15 Mesoporous Silica with Titanium. Chem. Mater. 2000, 12, 898–911. [Google Scholar] [CrossRef]
- Zhang, W.; Froba, M.; Wang, J.; Tanev, P.T.; Wong, J.; Pinnavaia, T.J. Mesoporous Titanosilicate Molecular Sieves Prepared at Ambient Temperature by Electrostatic (S+I−, S+X−I+) and Neutral (S°I°) Assembly Pathways: A Comparison of Physical Properties and Catalytic Activity for Peroxide Oxidations. J. Am. Chem. Soc. 1996, 118, 9164–9171. [Google Scholar] [CrossRef]
- Luan, Z.; Mases, E.M.; van der Heide, P.A.W.; Zhao, D.; Czernuszewicz, R.S.; Kevan, L. Incorporation of Titanium into Mesoporous Silica Molecular Sieve SBA-15. Chem. Mater. 1999, 11, 3680–3686. [Google Scholar] [CrossRef]
- Zhang, W.H.; Lu, J.; Han, B.; Li, M.; Xiu, J.; Ying, P.; Li, C. Direct Synthesis and Characterization of Titanium-Substituted Mesoporous Molecular Sieve SBA-15. Chem. Matter. 2002, 14, 3413–3421. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, X.S. Characterization and Photocatalytic Properties of Titanium-Containing Mesoporous SBA-15. Ind. Eng. Chem. Res. 2006, 45, 3569–3573. [Google Scholar]
- Morishita, M.; Shiraishi, Y.; Hirai, T. Ti-Containing Mesoporous Organosilica as a Photocatalyst for Selective Olefin Epoxidation. J. Phys. Chem. B 2006, 110, 17898–17905. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Morishita, M.; Hirai, T. Acetonitrile-assisted highly selective photocatalytic epoxidation of olefins on Ti-containing silica with molecular oxygen. Chem. Commun. 2005, 5977–5979. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ikeue, K.; Fujii, Y.; Zhang, S.G.; Ichihashi, Y.; Park, D.R.; Suzuki, Y.; Koyano, K.; Tatsumi, T. Photocatalytic reduction of CO2 with H2O on Ti-MCM-41 and Ti-MCM-48 mesoporous zeolite catalysts. Catal. Today 1998, 44, 327–332. [Google Scholar] [CrossRef]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent 4410501, 18 October 1983. [Google Scholar]
- Blasco, T.; Comblor, M.A.; Corma, A.; Pariente, J.P. The state of Ti in titanoaluminosilicates isomorphous with zeolite beta. J. Am. Chem. Soc. 1993, 115, 11806–11813. [Google Scholar] [CrossRef]
- Comblor, M.A.; Corma, A.; Martin, A.; Pariente, J.P. Synthesis of a titaniumsilicoaluminate isomorphous to zeolite beta and its application as a catalyst for the selective oxidation of large organic molecules. Chem. Commun. 1992, 589–590. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Raj, A. Synthesis of mesoporous hexagonal titanium aluminophosphate molecular sieves and their catalytic applications. Appl. Catal. 2000, 203, 311–319. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Bhaumik, A.; Inagaki, S.; Kuraoka, K.; Yazawa, T. Titanium containing inorganic–organic hybrid mesoporous materials with exceptional activity in epoxidation of alkenes using hydrogen peroxide. J. Mater. Chem. 2002, 12, 3078–3083. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Sinha, A.K.; Seelan, S.; Inagaki, S.; Tsubota, S.; Yoshida, H.; Haruta, M. Hydrophobicity induced vapor-phase oxidation of propene over gold supported on titanium incorporated hybrid mesoporous silsesquioxane. Chem. Commun. 2002, 2902–2903. [Google Scholar] [CrossRef]
- Bhaumik, A.; Kapoor, M.P.; Inagaki, S. Ammoximation of ketones catalyzed by titanium-containing ethane bridged hybrid mesoporous silsesquioxane. Chem. Commun. 2003, 470–471. [Google Scholar] [CrossRef]
- Ritterskamp, P.; Kuklya, A.; Wustkamp, M.A.; Kerpen, K.; Weidenthaler, C.; Demuth, M. A Titanium Disilicide Derived Semiconducting Catalyst for Water Splitting under Solar Radiation—Reversible Storage of Oxygen and Hydrogen. Angrew. Chem. Int. Ed. 2007, 46, 7770–7774. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Arsuaga, J.M.; Pardo, J.S.; Frutos, P.d.; Blazquez, S. Synthesis and catalytic activity of organic–inorganic hybrid Ti-SBA-15 materials. J. Mater. Chem. 2007, 17, 377–385. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous Organosilica with Amidoxime Groups for CO2 Sorption. Appl. Mater. Interfaces 2014, 6, 13069–13078. [Google Scholar] [CrossRef]
- Gunathilake, C.; Gorka, J.; Dai, S.; Jaroniec, M. Amidoxime-modified mesoporous silica for uranium adsorption under seawater conditions. J. Mater. Chem. A 2015, 3, 11650–11659. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous alumina–zirconia–organosilica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2015, 3, 2707–2716. [Google Scholar] [CrossRef]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. Mesoporous isocyanurate-containing organosilica–alumina composites and their thermal treatment in nitrogen for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A 2013, 1, 8244–8252. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of Large Pore MCM-41 Molecular Sieves to Improve Pore Size Analysis Using Nitrogen Adsorption Measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Kalpage, C.S.; Jaroniec, M. Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25 °C. Materials 2018, 11, 2301. [Google Scholar] [CrossRef]
- Gunathilake, C.; Manchanda, A.S.; Ghimire, P.; Kruk, M.M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: Synthesis and CO2 sorption properties. Environ. Sci. Nano 2016, 3, 806–817. [Google Scholar] [CrossRef]










| Sample Notation | Precursors | Condition | TiPO Moles | ICS Moles (x) | TEOS Moles (y) |
|---|---|---|---|---|---|
| Ti$ | TiPO | Extracted-Calcined at 350 °C | 0.011 | - | - |
| Ti* | TiPO | Calcined at 600 °C | 0.011 | - | - |
| Ti-Ix-TSy# | ICS, TEOS, TiPO | As-Synthesized | 0.011 | 0.011 (x)/100 | 0.011 (y)/100 |
| Ti-Ix | ICS, TiPO | Extracted-Calcined at 350 °C | 0.011 | 0.011 (x)/100 | - |
| Ti-Ix-TSy | ICS, TEOS, TiPO | Extracted-Calcined at 350 °C | 0.011 | 0.011 (x)/100 | 0.011 (y)/100 |
| Ti-Ix-TSy* | ICS, TEOS, TiPO | Calcined at 600 °C | 0.011 | 0.011 (x)/100 | 0.011 (y)/100 |
| Isotherm | Vsp (cm3/g) | Vmi (cm3/g) | SBET/(m2/g) | Wmax/nm | N (mmol/g) | C (mmol/g) | Initial Ti/Si Ratio |
|---|---|---|---|---|---|---|---|
| Ti-I5-TS5 | 0.45 | 0.01 | 275 | 7.6 | 0.35 | 7.52 | 10:1 |
| Ti-I10-TS10 | 0.50 | 0.01 | 264 | 7.3 | 0.65 | 8.38 | 10:2 |
| Ti-I20 | 0.40 | 0.01 | 160 | 5.8 | 1.09 | 8.42 | 10:2 |
| Ti-I50-TS50 | 0.39 | 0.04 | 334 | 4.0 | 1.80 | 13.90 | 10:10 |
| Ti-I100 | 0.44 | 0.03 | 269 | 3.2 | 3.42 | 18.69 | 10:10 |
| Ti$ | 0.36 | 0.01 | 144 | 9.2 | - | - | - |
| Ti-I10-TS10 | 0.50 | 0.01 | 264 | 7.3 | 0.65 | 8.38 | 10:2 |
| Ti-I10-TS30 | 0.51 | 0.02 | 332 | 7.3 | 0.60 | 7.21 | 10:4 |
| Ti-I10-TS50 | 0.46 | 0.03 | 371 | 5.6 | 0.59 | 9.06 | 10:6 |
| Ti-I10-TS90 | 0.53 | 0.05 | 475 | 4.8 | 0.52 | 9.88 | 10:10 |
| Ti* | 0.36 | 0.01 | 111 | 11.6 | - | - | - |
| Ti-I10-TS10* | 0.43 | 0.01 | 225 | 8.0 | 0.17 | 5.44 | 10:2 |
| Ti-I10-TS30* | 0.46 | 0.01 | 300 | 7.3 | 0.14 | 4.69 | 10:4 |
| Ti-I10-TS50* | 0.45 | 0.03 | 373 | 5.0 | 0.15 | 6.40 | 10:6 |
| Ti-I10-TS90* | 0.73 | 0.04 | 487 | 4.6 | 0.15 | 6.29 | 10:10 |
| Sample | SBET (m2/g) | N (mmol/g) | n CO2 (mmol/g) | n*CO2 (µmol/m2) | ASA (m2/g) | 100.ASA/SBET (%) |
|---|---|---|---|---|---|---|
| Ti-I5-TS5 | 275 | 0.35 | 0.70 | 2.54 | 91.9 | 33.4 |
| Ti-I10-TS10 | 264 | 0.65 | 0.63 | 2.38 | 82.7 | 31.3 |
| Ti-I20 | 160 | 1.09 | 0.60 | 3.75 | 78.8 | 49.2 |
| Ti-I50-TS50 | 334 | 1.80 | 0.59 | 1.76 | 77.4 | 23.2 |
| Ti-I100 | 269 | 3.42 | 0.55 | 2.05 | 72.2 | 26.9 |
| Ti$ | 144 | - | 0.62 | 4.30 | 81.4 | 56.5 |
| Ti-I10-TS10 | 264 | 0.65 | 0.63 | 2.38 | 82.7 | 31.3 |
| Ti-I10-TS30 | 332 | 0.60 | 0.64 | 1.93 | 84.0 | 25.3 |
| Ti-I10-TS50 | 371 | 0.59 | 0.69 | 1.86 | 90.6 | 24.4 |
| Ti-I10-TS90 | 475 | 0.52 | 0.72 | 1.52 | 94.5 | 19.9 |
| Ti* | 111 | - | 0.41 | 3.68 | 53.8 | 48.3 |
| Ti-I10-TS10* | 225 | 0.17 | 0.48 | 2.13 | 63.0 | 28.0 |
| Ti-I10-TS30* | 300 | 0.14 | 0.68 | 2.27 | 89.3 | 29.8 |
| Ti-I10-TS50* | 373 | 0.15 | 0.72 | 1.93 | 94.5 | 25.3 |
| Ti-I10-TS90* | 487 | 0.15 | 0.81 | 1.66 | 106.3 | 21.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilake, C.; Kalpage, C.; Kadanapitiye, M.; Dassanayake, R.S.; Manchanda, A.S.; Gangoda, M. Facile Synthesis and Surface Characterization of Titania-Incorporated Mesoporous Organosilica Materials. J. Compos. Sci. 2019, 3, 77. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs3030077
Gunathilake C, Kalpage C, Kadanapitiye M, Dassanayake RS, Manchanda AS, Gangoda M. Facile Synthesis and Surface Characterization of Titania-Incorporated Mesoporous Organosilica Materials. Journal of Composites Science. 2019; 3(3):77. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs3030077
Chicago/Turabian StyleGunathilake, Chamila, Chandrakantha Kalpage, Murthi Kadanapitiye, Rohan S. Dassanayake, Amanpreet S. Manchanda, and Mahinda Gangoda. 2019. "Facile Synthesis and Surface Characterization of Titania-Incorporated Mesoporous Organosilica Materials" Journal of Composites Science 3, no. 3: 77. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs3030077